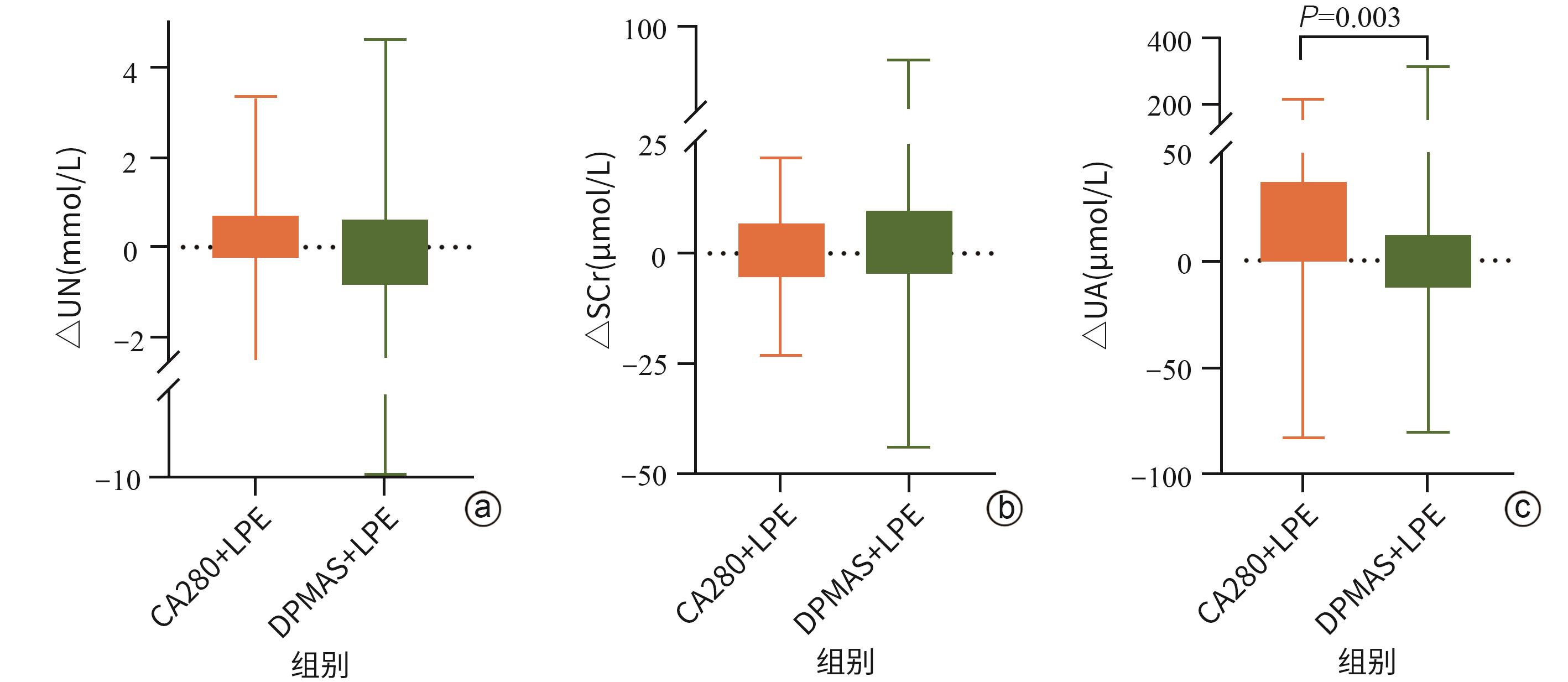
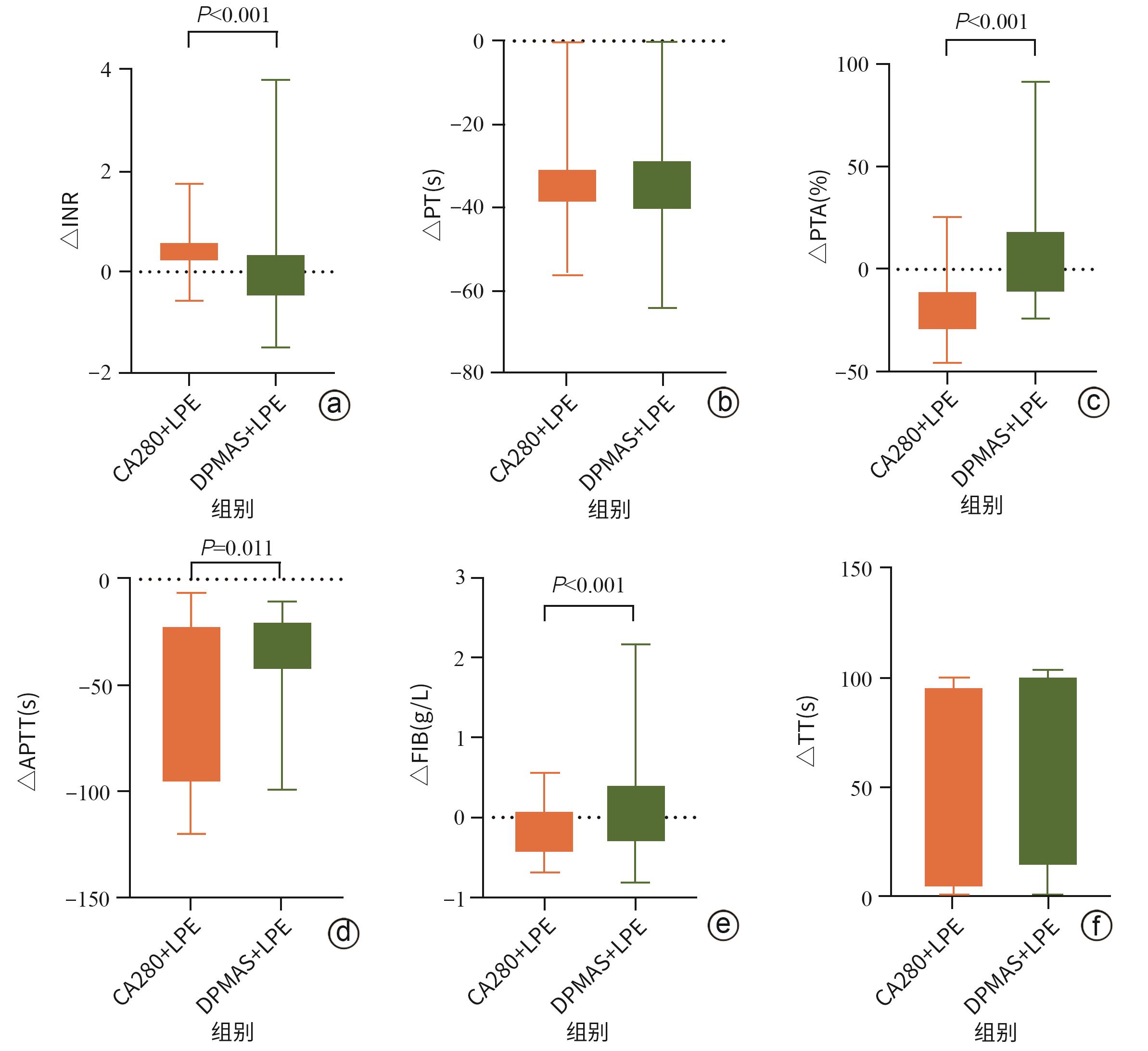
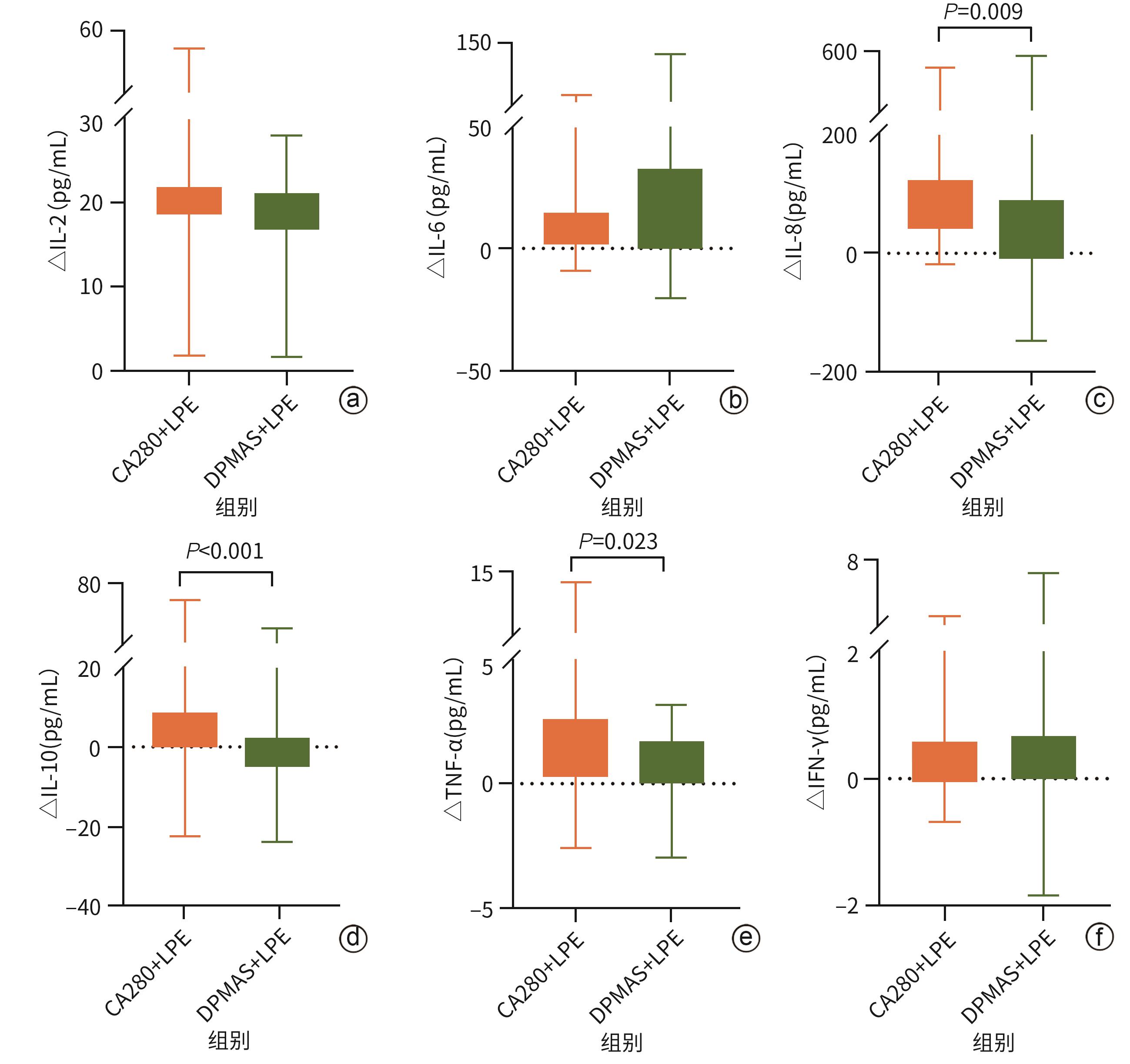
| [1] |
BR VK, SARIN SK. Acute-on-chronic liver failure: Terminology, mechanisms and management[J]. Clin Mol Hepatol, 2023, 29( 3): 670- 689. DOI: 10.3350/cmh.2022.0103. |
| [2] |
CASULLERAS M, ZHANG IW, LÓPEZ-VICARIO C, et al. Leukocytes, systemic inflammation and immunopathology in acute-on-chronic liver failure[J]. Cells, 2020, 9( 12): 2632. DOI: 10.3390/cells9122632. |
| [3] |
HEO SK, YU HM, KIM DK, et al. LIGHT(TNFSF14) promotes the differentiation of human bone marrow-derived mesenchymal stem cells into functional hepatocyte-like cells[J]. PLoS One, 2023, 18( 8): e0289798. DOI: 10.1371/journal.pone.0289798. |
| [4] |
ZHU BB, GAO FY, LI YX, et al. Serum cytokine and chemokine profiles and disease prognosis in hepatitis B virus-related acute-on-chronic liver failure[J]. Front Immunol, 2023, 14: 1133656. DOI: 10.3389/fimmu.2023.1133656. |
| [5] |
YE C, LI WY, LI L, et al. Glucocorticoid treatment strategies in liver failure[J]. Front Immunol, 2022, 13: 846091. DOI: 10.3389/fimmu.2022.846091. |
| [6] |
QIANG R, LIU XZ, XU JC. The immune pathogenesis of acute-on-chronic liver failure and the danger hypothesis[J]. Front Immunol, 2022, 13: 935160. DOI: 10.3389/fimmu.2022.935160. |
| [7] |
FERNÁNDEZ J, ACEVEDO J, WIEST R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis[J]. Gut, 2018, 67( 10): 1870- 1880. DOI: 10.1136/gutjnl-2017-314240. |
| [8] |
MANTOVANI A, DINARELLO CA, MOLGORA M, et al. Interleukin-1 and related cytokines in the regulation of inflammation and immunity[J]. Immunity, 2019, 50( 4): 778- 795. DOI: 10.1016/j.immuni.2019.03.012. |
| [9] |
DU LY, MA YJ, ZHOU SQ, et al. A prognostic score for patients with acute-on-chronic liver failure treated with plasma exchange-centered artificial liver support system[J]. Sci Rep, 2021, 11( 1): 1469. DOI: 10.1038/s41598-021-81019-8. |
| [10] |
ZHANG Z, ZHU J, DOU Y. Effect of artificial liver plasma exchange combined with CRRT in the treatment of hepatitis B-related chronic acute liver failure complicated with acute renal failure and its impact on prognosis[J]. Clin Misdiagn Misther, 2023, 36( 10): 86- 90. DOI: 10.3969/j.issn.1002-3429.2023.10.019. |
| [11] |
WU CC, PENG WT, CHENG D, et al. Efficacy and economic evaluation of nonbiological artificial liver therapy in acute-on-chronic hepatitis B liver failure[J]. J Clin Transl Hepatol, 2023, 11( 2): 433- 440. DOI: 10.14218/JCTH.2022.00106. |
| [12] |
SHANG J, WANG MQ, WEN Q, et al. A novel prognostic model to predict outcome of artificial liver support system treatment[J]. Sci Rep, 2021, 11( 1): 7510. DOI: 10.1038/s41598-021-87055-8. |
| [13] |
HUANG YD, JU T, ZHANG HF, et al. Lower level of IL-28A as a predictive index of the artificial liver support system in effective treatment of patients with HBV-ACLF[J]. J Clin Lab Anal, 2022, 36( 12): e24766. DOI: 10.1002/jcla.24766. |
| [14] |
WANG L, XU WX, ZHU S, et al. Double plasma molecular adsorption system with sequential low-dose plasma exchange in patients with hepatitis B virus-related acute-on-chronic liver failure: A prospective study[J]. J Clin Transl Hepatol, 2023, 11( 4): 908- 917. DOI: 10.14218/JCTH.2022.00254. |
| [15] |
HE J, ZHANG XP, ZHOU X, et al. Application of double plasma molecular adsorption system in children with acute liver failure[J]. Chin J Contemp Pediatr, 2021, 23( 2): 180- 185. DOI: 10.7499/j.issn.1008-8830.2010145. |
| [16] |
SEKANDARZAD A, WEBER E, PRAGER EP, et al. Cytokine adsorption in patients with acute-on-chronic liver failure(CYTOHEP)-a single center, open-label, three-arm, randomized, controlled intervention trial[J]. Trials, 2022, 23( 1): 222. DOI: 10.1186/s13063-022-06139-6. |
| [17] |
AGARWAL B, CAÑIZARES RB, SALIBA F, et al. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure[J]. J Hepatol, 2023, 79( 1): 79- 92. DOI: 10.1016/j.jhep.2023.03.013. |
| [18] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2024 version)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. 中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. |
| [19] |
ZENG YY, GAN DK, ZHANG KG, et al. The impact of artificial liver support system on intestinal microbiota and serum bile acid profiles in patients with acute-on-chronic liver failure: A prospective cohort study[J]. Hepatol Int, 2024, 18( 5): 1540- 1554. DOI: 10.1007/s12072-024-10712-3. |
| [20] |
BATEMAN RM, SHARPE MD, JAGGER JE, et al. 36th international symposium on intensive care and emergency medicine: Brussels, Belgium. 15-18 March 2016[J]. Crit Care, 2016, 20( Suppl 2): 94. DOI: 10.1186/s13054-016-1208-6. |
| [21] |
RONCO C, CHAWLA L, HUSAIN-SYED F, et al. Rationale for sequential extracorporeal therapy(SET) in sepsis[J]. Crit Care, 2023, 27( 1): 50. DOI: 10.1186/s13054-023-04310-2. |
| [22] |
RADMANIC MATOTEK L, ZIDOVEC-LEPEJ S, SALEK N, et al. The impact of liver steatosis on interleukin and growth factors kinetics during chronic hepatitis C treatment[J]. J Clin Med, 2024, 13( 16): 4849. DOI: 10.3390/jcm13164849. |
| [23] |
ZHOU C, ZHANG N, HE TT, et al. High levels of serum interleukin-6 increase mortality of hepatitis B virus-associated acute-on-chronic liver failure[J]. World J Gastroenterol, 2020, 26( 30): 4479- 4488. DOI: 10.3748/wjg.v26.i30.4479. |
| [24] |
WU ZB, ZHENG YB, WANG K, et al. Plasma interleukin-6 level: A potential prognostic indicator of emergent HBV-associated ACLF[J]. Can J Gastroenterol Hepatol, 2021, 2021: 5545181. DOI: 10.1155/2021/5545181. |
| [25] |
TARU V, SZABO G, MEHAL W, et al. Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation[J]. J Hepatol, 2024, 81( 5): 895- 910. DOI: 10.1016/j.jhep.2024.06.016. |
| [26] |
SCHWARZKOPF K, RÜSCHENBAUM S, BARAT S, et al. IL-22 and IL-22-binding protein are associated with development of and mortality from acute-on-chronic liver failure[J]. Hepatol Commun, 2019, 3( 3): 392- 405. DOI: 10.1002/hep4.1303. |
| [27] |
XIE ZB, DING L, LI YZ. Computed tomography image features under convolutional neural network algorithm in analysis of inflammatory factor level and prognosis of patients with hepatitis B virus-associated acute-on-chronic liver failure[J]. J Healthc Eng, 2021, 2021: 2110612. DOI: 10.1155/2021/2110612. |
| [28] |
MOOKERJEE RP. Prognosis and biomarkers in acute-on-chronic liver failure[J]. Semin Liver Dis, 2016, 36( 2): 127- 132. DOI: 10.1055/s-0036-1583200. |



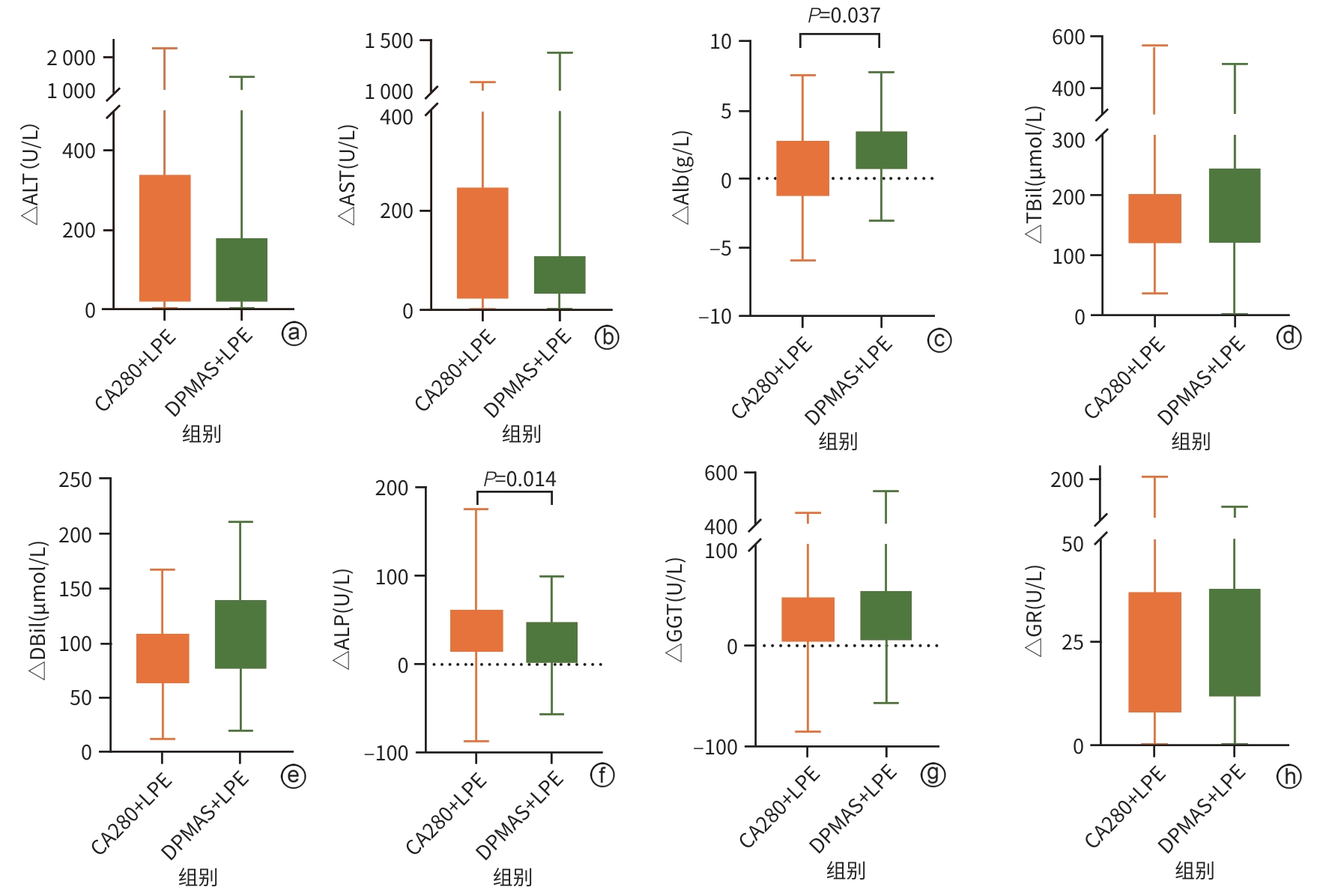




 DownLoad:
DownLoad: