| [1] |
Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study[J]. Lancet Gastroenterol Hepatol, 2022, 7( 5): 396- 415. DOI: 10.1016/S2468-1253(21)00472-6. |
| [2] |
Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infections Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045. |
| [3] |
Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study[J]. Lancet Gastroenterol Hepatol, 2017, 2( 3): 161- 176. DOI: 10.1016/S2468-1253(16)30181-9. |
| [4] |
MESSINA JP, HUMPHREYS I, FLAXMAN A, et al. Global distribution and prevalence of hepatitis C virus genotypes[J]. Hepatology, 2015, 61( 1): 77- 87. DOI: 10.1002/hep.27259. |
| [5] |
RAO HY, WEI L, LOPEZ-TALAVERA JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection[J]. J Gastroenterol Hepatol, 2014, 29( 3): 545- 553. DOI: 10.1111/jgh.12398. |
| [6] |
TANG Q, CHEN ZW, LI H, et al. Molecular epidemiology of hepatitis C virus genotypes in different geographical regions of Chinese mainland and a phylogenetic analysis[J]. Infect Dis Poverty, 2023, 12( 1): 66. DOI: 10.1186/s40249-023-01106-y. |
| [7] |
CHEN Y, YU CS, YIN XR, et al. Hepatitis C virus genotypes and subtypes circulating in Mainland China[J]. Emerg Microbes Infect, 2017, 6( 11): e95. DOI: 10.1038/emi.2017.77. |
| [8] |
LI F, LI B, ZHU QY. Research progress on the epidemiological characteristics and diagnosis of hepatitis C in China[J]. Int J Virol, 2023, 30( 6): 509- 511. DOI: 10.3760/cma.j.issn.1673-4092.2023.06.014. |
| [9] |
BOJANIC K, BOGOJEVIC MS, VUKADIN S, et al. Noninvasive fibrosis assessment in chronic hepatitis C infection: An update[J]. J Clin Transl Hepatol, 2023, 11( 5): 1228- 1238. DOI: 10.14218/JCTH.2022.00365. |
| [10] |
JI FP, LI J, LIU L, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b[J]. J Gastroenterol Hepatol, 2021, 36( 3): 767- 774. DOI: 10.1111/jgh.15192. |
| [11] |
HUANG CF, IIO E, JUN DW, et al. Direct-acting antivirals in East Asian hepatitis C patients: Real-world experience from the REAL-C Consortium[J]. Hepatol Int, 2019, 13( 5): 587- 598. DOI: 10.1007/s12072-019-09974-z. |
| [12] |
HUANG R, RAO HY, XIE Q, et al. Comparison of the efficacy of sofosbuvir plus ribavirin in Chinese patients with genotype 3a or 3b HCV infection[J]. J Med Virol, 2019, 91( 7): 1313- 1318. DOI: 10.1002/jmv.25454. |
| [13] |
WU N, RAO HY, YANG WB, et al. Impact of hepatitis C virus genotype 3 on liver disease progression in a Chinese national cohort[J]. Chin Med J(Engl), 2020, 133( 3): 253- 261. DOI: 10.1097/CM9.0000000000000629. |
| [14] |
DEGENHARDT L, PEACOCK A, COLLEDGE S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review[J]. Lancet Glob Health, 2017, 5( 12): e1192- e1207. DOI: 10.1016/S2214-109X(17)30375-3. |
| [15] |
ADINOLFI LE, UTILI R, ANDREANA A, et al. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C[J]. Dig Dis Sci, 2001, 46( 8): 1677- 1683. DOI: 10.1023/a:1010697319589. |
| [16] |
LIU CR, LI X, CHAN PL, et al. Prevalence of hepatitis C virus infection among key populations in China: A systematic review[J]. Int J Infect Dis, 2019, 80: 16- 27. DOI: 10.1016/j.ijid.2018.11.006. |
| [17] |
HAQUE LY, FIELLIN DA, TATE JP, et al. Association between alcohol use disorder and receipt of direct-acting antiviral hepatitis C virus treatment[J]. JAMA Netw Open, 2022, 5( 12): e2246604. DOI: 10.1001/jamanetworkopen.2022.46604. |
| [18] |
IM PK, MILLWOOD IY, KARTSONAKI C, et al. Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: A 10-year prospective study of 0.5 million adults[J]. BMC Med, 2021, 19( 1): 216. DOI: 10.1186/s12916-021-02079-1. |
| [19] |
PREMKUMAR M, ANAND AC. Tobacco, cigarettes, and the liver: The smoking Gun[J]. J Clin Exp Hepatol, 2021, 11( 6): 700- 712. DOI: 10.1016/j.jceh.2021.07.016. |
| [20] |
WEI L, LIM SG, XIE Q, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: A single-arm, open-label, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2019, 4( 2): 127- 134. DOI: 10.1016/S2468-1253(18)30343-1. |
| [21] |
JI FP, TRAN S, OGAWA E, et al. Real-world effectiveness and tolerability of interferon-free direct-acting antiviral for 15, 849 patients with chronic hepatitis C: A multinational cohort study[J]. J Clin Transl Hepatol, 2024, 12( 7): 646- 658. DOI: 10.14218/JCTH.2024.00089. |
| [22] |
ABULITIFU YLHM, LIAN JS, BALI RML, et al. Efficacy and safety of Sofosbuvir/Velpatasvir or combined with Ribavirin in treatment of patients with hepatitis C related cirrhosis in Xinjiang region[J]. Chin J Clin Infect Dis, 2022, 15( 6): 459- 463. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.005. |
| [23] |
LIU YY, LIU N, FU JJ, et al. Distribution characteristics of hepatitis C virus genotypes in Shaanxi Province[J]. Hainan Med J, 2021, 32( 4): 413- 416. DOI: 10.3969/j.issn.1003-6350.2021.04.002. |
| [24] |
ZHANG HY, RAO HY, CHEN HS. Current status of hepatitis C virus infection and progress in its elimination in China[J]. J Clin Hepatol, 2024, 40( 4): 649- 653. DOI: 10.12449/JCH240401. 张海莹, 饶慧瑛, 陈红松. 中国丙型肝炎病毒感染的现状及清除进程[J]. 临床肝胆病杂志, 2024, 40( 4): 649- 653. DOI: 10.12449/JCH240401. |



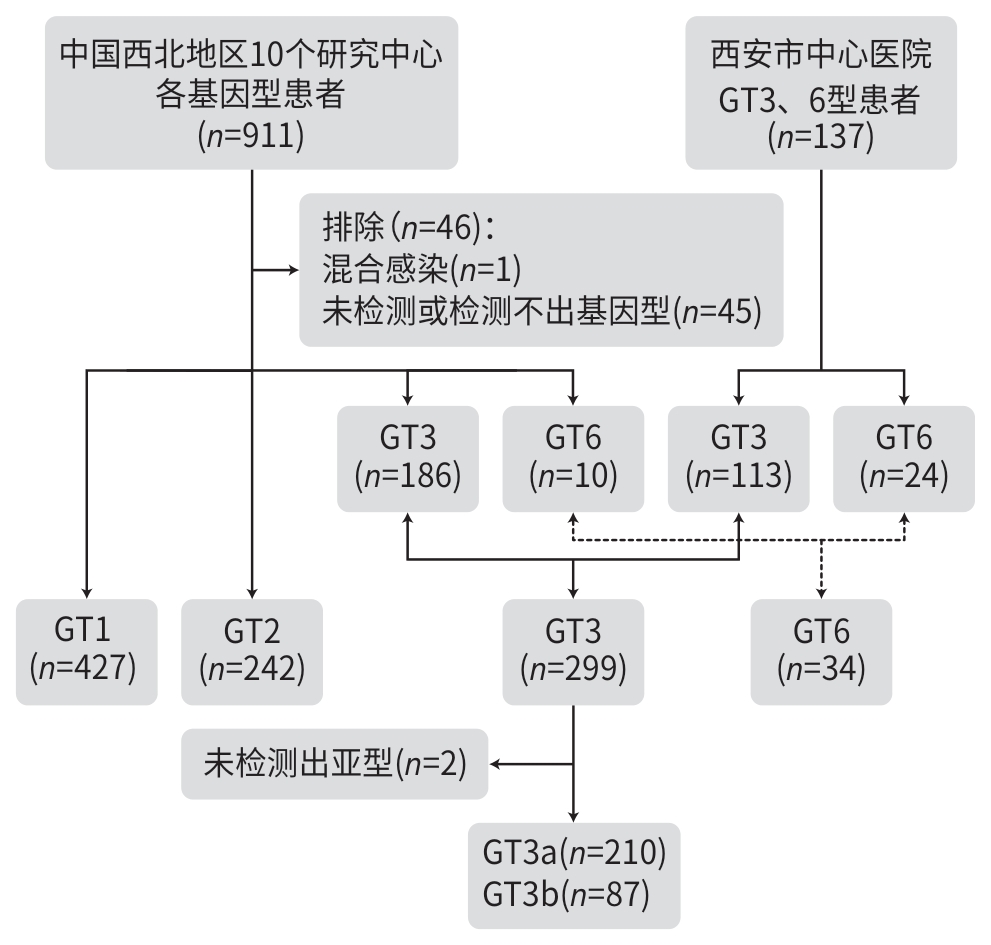




 DownLoad:
DownLoad: