| [1] |
STASI C, SILVESTRI C, VOLLER F. Hepatitis B vaccination and immunotherapies: an update[J]. Clin Exp Vaccine Res, 2020, 9( 1): 1- 7. DOI: 10.7774/cevr.2020.9.1.1. |
| [2] |
SHAN S, ZHAO XY, JIA JD. Comprehensive approach to controlling chronic hepatitis B in China[J]. Clin Mol Hepatol, 2024, 30( 2): 135- 143. DOI: 10.3350/cmh.2023.0412. |
| [3] |
CAO GY, LIU J, LIU M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: Findings from the global burden of disease study[J]. Chin Med J(Engl), 2022, 135( 17): 2049- 2055. DOI: 10.1097/CM9.0000000000002331. |
| [4] |
POLLICINO T, CAMINITI G. HBV-integration studies in the clinic: Role in the natural history of infection[J]. Viruses, 2021, 13( 3): 368. DOI: 10.3390/v13030368. |
| [5] |
ZHUANG H. Debates on the natural history of chronic hepatitis B virus infection during the update of Guidelines for the Prevention and Treatment of Chronic Hepatitis B(2022 Version)[J]. J Clin Hepatol, 2023, 39( 6): 1295- 1298. DOI: 10.3969/j.issn.1001-5256.2023.06.006. |
| [6] |
CHU CM, LIAW YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: Appreciably high rates during a long-term follow-up[J]. Hepatology, 2007, 45( 5): 1187- 1192. DOI: 10.1002/hep.21612. |
| [7] |
HUANG Y, QI M, LIAO CJ, et al. Analysis of the efficacy and safety of PEGylated interferon-α2b treatment in inactive hepatitis B surface antigen carriers[J]. Infect Dis Ther, 2021, 10( 4): 2323- 2331. DOI: 10.1007/s40121-021-00511-w. |
| [8] |
DUAN SP, ZHU LH, HOU LJ, et al. Effect of tenofovir disoproxil fumarate antiviral therapy on virus-specific CD8 +T Cells function in patients with chronic hepatitis B[J]. Chin J Hepatol, 2021, 29( 5): 421- 426. DOI: 10.3760/cma.j.cn501113-20191113-00420. |
| [9] |
WAN LH, ZHANG J, CHEN S, et al. Influnces of tenofovir dipivoxil combined with compound Yiganling tablets on immune function, liver and kidney function in HBV carrier complicated with pulmonary tuberculosis[J]. J Clin Exp Med, 2023, 22( 18): 1938- 1942. DOI: 10.3969/j.issn.1671-4695.2023.18.010. |
| [10] |
DONG J, YANG XF, WANG LX, et al. Modulation of tim-3 expression by antigen-dependent and-independent factors on T cells from patients with chronic hepatitis B virus infection[J]. Front Cell Infect Microbiol, 2017, 7: 98. DOI: 10.3389/fcimb.2017.00098. |
| [11] |
McLANE LM, ABDEL-HAKEEM MS, WHERRY EJ. CD8 T cell exhaustion during chronic viral infection and cancer[J]. Annu Rev Immunol, 2019, 37: 457- 495. DOI: 10.1146/annurev-immunol-041015-055318. |
| [12] |
WONG GLH, GANE E, LOK ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development?[J]. J Hepatol, 2022, 76( 6): 1249- 1262. DOI: 10.1016/j.jhep.2021.11.024. |
| [13] |
DENG W, JIANG TT, BI XY, et al. Progress on the treatment of chronic hepatitis B with interferons[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 2): 1- 6. DOI: 10.3969/j.issn.1674-7380.2023.02.001. |
| [14] |
ISLAM M, KUMAR K, SEVAK JK, et al. Immune drivers of HBsAg loss in HBeAg-negative CHB patients after stopping nucleotide analog and administration of Peg-IFN[J]. Hepatol Commun, 2023, 7( 5): e0098. DOI: 10.1097/HC9.0000000000000098. |
| [15] |
PANG XQ, LI X, ZHU WH, et al. LAG3 + erythroid progenitor cells inhibit HBsAg seroclearance during finite pegylated interferon treatment through LAG3 and TGF-β[J]. Antiviral Res, 2023, 213: 105592. DOI: 10.1016/j.antiviral.2023.105592. |
| [16] |
HUANG D, YAN WM, HAN MF, et al. Insufficient immunity led to virologic breakthrough in NAs-treated chronic hepatitis B patients switching to Peg-IFN-α[J]. Antiviral Res, 2022, 197: 105220. DOI: 10.1016/j.antiviral.2021.105220. |
| [17] |
WANG DY, FU BQ, SHEN XK, et al. Restoration of HBV-specific CD8 + T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy[J]. Signal Transduct Target Ther, 2021, 6( 1): 376. DOI: 10.1038/s41392-021-00776-0. |
| [18] |
MONTANARI NR, CONCEIÇÃO-NETO N, van den WYNGAERT I, et al. Differential gene expression, irrespective of circulating hepatitis B surface antigen levels, between inactive carrier and nucleos(t)ide analogue-treated hepatitis B virus patients[J]. J Infect Dis, 2022, 225( 8): 1471- 1476. DOI: 10.1093/infdis/jiaa614. |
| [19] |
SONG AX, LIN X, LU JF, et al. Pegylated interferon treatment for the effective clearance of hepatitis B surface antigen in inactive HBsAg carriers: A meta-analysis[J]. Front Immunol, 2021, 12: 779347. DOI: 10.3389/fimmu.2021.779347. |
| [20] |
CHEN XB, LIU FF, SHU FL, et al. Peginterferon Alfa-2b combined with tenofovir disoproxil fumarate induced high clinical cure rate in inactive chronic hepatitis B virus carriers[J]. Clin Res Hepatol Gastroenterol, 2021, 45( 5): 101723. DOI: 10.1016/j.clinre.2021.101723. |
| [21] |
CAO ZH, LIU YL, MA LN, et al. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha[J]. Hepatology, 2017, 66( 4): 1058- 1066. DOI: 10.1002/hep.29213. |
| [22] |
ZHU L, LI J, XU JC, et al. Significance of T-cell subsets for clinical response to peginterferon Alfa-2a therapy in HBeAg-positive chronic hepatitis B patients[J]. Int J Gen Med, 2022, 15: 4441- 4451. DOI: 10.2147/IJGM.S356696. |
| [23] |
PENG MJ, GUO XQ, ZHANG WL, et al. Effect of pegylated interferon-α2b add-on therapy on renal function in chronic hepatitis B patients: A real-world experience[J]. Front Microbiol, 2022, 13: 980250. DOI: 10.3389/fmicb.2022.980250. |
| [24] |
RASKOV H, ORHAN A, CHRISTENSEN JP, et al. Cytotoxic CD8 + T cells in cancer and cancer immunotherapy[J]. Br J Cancer, 2021, 124( 2): 359- 367. DOI: 10.1038/s41416-020-01048-4. |
| [25] |
VERDON DJ, MULAZZANI M, JENKINS MR. Cellular and molecular mechanisms of CD8 + T cell differentiation, dysfunction and exhaustion[J]. Int J Mol Sci, 2020, 21( 19): 7357. DOI: 10.3390/ijms21197357. |
| [26] |
PHILLIPS S, CHOKSHI S, RIVA A, et al. CD8 + T cell control of hepatitis B virus replication: Direct comparison between cytolytic and noncytolytic functions[J]. J Immunol, 2010, 184( 1): 287- 295. DOI: 10.4049/jimmunol.0902761. |
| [27] |
SHAO X, MA JT, JIA SN, et al. Interleukin-35 suppresses antiviral immune response in chronic hepatitis B virus infection[J]. Front Cell Infect Microbiol, 2017, 7: 472. DOI: 10.3389/fcimb.2017.00472. |
| [28] |
Chinese Society of Hepatology, Chinese Society of Infectious Diseases. Guidelines for the prevention and treatment of chronic hepatitis B(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050. |



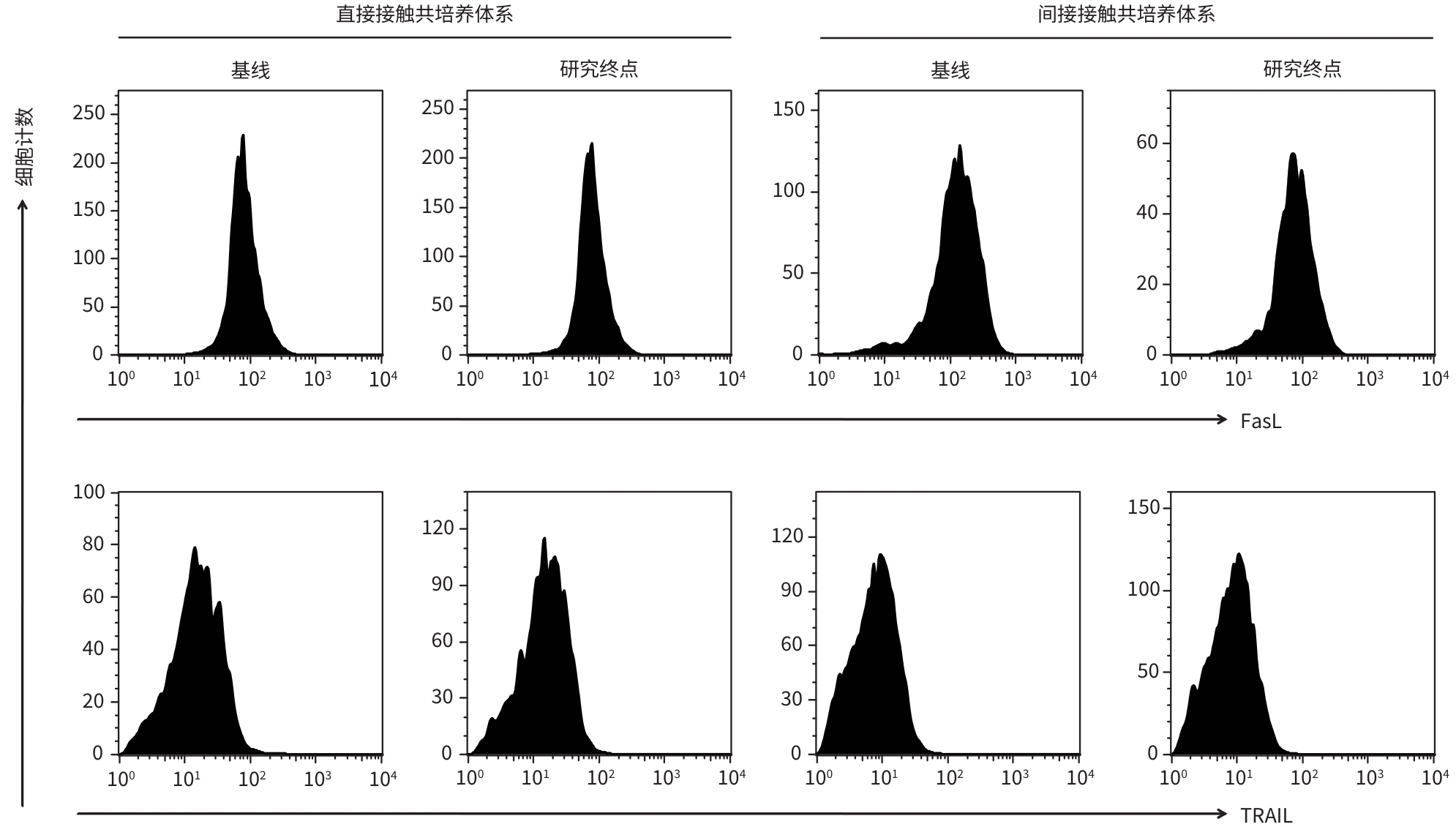




 DownLoad:
DownLoad: