程序性细胞死亡受体1与细胞毒性T淋巴细胞相关抗原4联合阻断治疗胰腺癌的研究进展
DOI: 10.12449/JCH251233
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:秦文科、赵学安负责综述框架设计,文献检索与筛选,主体内容撰写;魏孔源参与收集数据,修改论文;张辉、周文策负责拟定写作思路,指导撰写文章并最后定稿。
Research advances in combined blockade therapy for programmed cell death-1 and cytotoxic T-lymphocyte-associated antigen 4 in pancreatic cancer
-
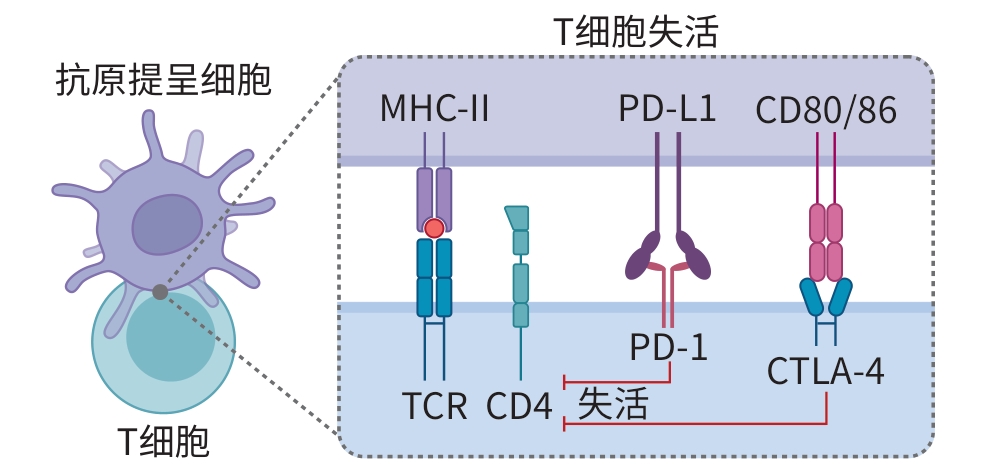
摘要: 胰腺癌(PC)是恶性程度极高、预后极差的消化道肿瘤,传统治疗手段对延长患者生存期的效果有限。近年来,免疫检查点抑制剂在多种实体瘤中取得突破性进展,其中程序性细胞死亡受体1与细胞毒性T淋巴细胞相关抗原4作为关键免疫检查点靶点备受关注。尽管二者在PC中的单独应用疗效不太理想,但双重阻断策略展现出更大的治疗潜力。本文从PC免疫微环境出发,系统综述程序性细胞死亡受体1与细胞毒性T淋巴细胞相关抗原4的生物学特性及其单药应用现状,重点探讨双重靶向治疗在PC中的研究进展及面临的挑战,并对未来发展方向进行展望。
-
关键词:
- 胰腺肿瘤 /
- 免疫检查点抑制剂 /
- 程序性细胞死亡受体1 /
- CTLA-4抗原
Abstract: Pancreatic cancer is a highly malignant tumor of the digestive system with an extremely poor prognosis, and traditional treatment methods have a limited effect in prolonging the survival time of patients. In recent years, ground-breaking advances have been achieved for immune checkpoint inhibitors (ICI) in a variety of solid tumors, among which programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) have attracted much attention as major immune checkpoint targets. Although single-agent treatment with PD-1 or CTLA-4 inhibitor has an unsatisfactory effect in PC, the strategy of dual blockade has shown greater therapeutic potential. Starting from the immune microenvironment of PC, this article systematically reviews the biological characteristics of PD-1 and CTLA-4 and the current status of their single-agent applications, discusses the research advances and challenges in dual-targeted therapy for PC, and proposes the prospects for future development in this field. -
[1] HALBROOK CJ, LYSSIOTIS CA, PASCA DI MAGLIANO M, et al. Pancreatic cancer: Advances and challenges[J]. Cell, 2023, 186( 8): 1729- 1754. DOI: 10.1016/j.cell.2023.02.014. [2] HU Z, O'REILLY EM. Therapeutic developments in pancreatic cancer[J]. Nat Rev Gastroenterol Hepatol, 2024, 21( 1): 7- 24. DOI: 10.1038/s41575-023-00840-w. [3] KOLBEINSSON HM, CHANDANA S, WRIGHT GP, et al. Pancreatic cancer: A review of current treatment and novel therapies[J]. J Invest Surg, 2023, 36( 1): 2129884. DOI: 10.1080/08941939.2022.2129884. [4] CHENG WS, KANG K, ZHAO AL, et al. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer[J]. J Hematol Oncol, 2024, 17( 1): 54. DOI: 10.1186/s13045-024-01581-2. [5] CHEN DS, MELLMAN I. Oncology meets immunology: The cancer-immunity cycle[J]. Immunity, 2013, 39( 1): 1- 10. DOI: 10.1016/j.immuni.2013.07.012. [6] VINAY DS, RYAN EP, PAWELEC G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies[J]. Semin Cancer Biol, 2015, 35 Suppl: S185- S198. DOI: 10.1016/j.semcancer.2015.03.004. [7] BUCHBINDER EI, DESAI A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition[J]. Am J Clin Oncol, 2016, 39( 1): 98- 106. DOI: 10.1097/COC.0000000000000239. [8] TOPALIAN SL, DRAKE CG, PARDOLL DM. Immune checkpoint blockade: A common denominator approach to cancer therapy[J]. Cancer Cell, 2015, 27( 4): 450- 461. DOI: 10.1016/j.ccell.2015.03.001. [9] FARES CM, van ALLEN EM, DRAKE CG, et al. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients?[J]. Am Soc Clin Oncol Educ Book, 2019, 39: 147- 164. DOI: 10.1200/EDBK_240837. [10] PASSARO A, BRAHMER J, ANTONIA S, et al. Managing resistance to immune checkpoint inhibitors in lung cancer: Treatment and novel strategies[J]. J Clin Oncol, 2022, 40( 6): 598- 610. DOI: 10.1200/JCO.21.01845. [11] WEI SC, LEVINE JH, COGDILL AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade[J]. Cell, 2017, 170( 6): 1120- 1133. e 17. DOI: 10.1016/j.cell.2017.07.024. [12] KVISTBORG P, PHILIPS D, KELDERMAN S, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response[J]. Sci Transl Med, 2014, 6( 254): 254ra128. DOI: 10.1126/scitranslmed.3008918. [13] LEACH DR, KRUMMEL MF, ALLISON JP. Enhancement of antitumor immunity by CTLA-4 blockade[J]. Science, 1996, 271( 5256): 1734- 1736. DOI: 10.1126/science.271.5256.1734. [14] WONG RM, SCOTLAND RR, LAU RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs[J]. Int Immunol, 2007, 19( 10): 1223- 1234. DOI: 10.1093/intimm/dxm091. [15] WEI SC, ANANG NAS, SHARMA R, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies[J]. Proc Natl Acad Sci USA, 2019, 116( 45): 22699- 22709. DOI: 10.1073/pnas.1821218116. [16] SUN T, ZHANG WJ, LI Y, et al. Combination immunotherapy with cytotoxic T-lymphocyte-associated antigen-4 and programmed death protein-1 inhibitors prevents postoperative breast tumor recurrence and metastasis[J]. Mol Cancer Ther, 2020, 19( 3): 802- 811. DOI: 10.1158/1535-7163.MCT-19-0495. [17] YEO J, KO M, LEE DH, et al. TIGIT/CD226 axis regulates anti-tumor immunity[J]. Pharmaceuticals, 2021, 14( 3): 200. DOI: 10.3390/ph14030200. [18] CURRAN MA, MONTALVO W, YAGITA H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors[J]. Proc Natl Acad Sci USA, 2010, 107( 9): 4275- 4280. DOI: 10.1073/pnas.0915174107. [19] PARDOLL DM. The blockade of immune checkpoints in cancer immunotherapy[J]. Nat Rev Cancer, 2012, 12( 4): 252- 264. DOI: 10.1038/nrc3239. [20] LI KY, TANDURELLA JA, GAI J, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy[J]. Cancer Cell, 2022, 40( 11): 1374- 1391. e 7. DOI: 10.1016/j.ccell.2022.10.001. [21] BRAHMER JR, DRAKE CG, WOLLNER I, et al. Phase I study of single-agent anti-programmed death-1(MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates[J]. J Clin Oncol, 2010, 28( 19): 3167- 3175. DOI: 10.1200/JCO.2009.26.7609. [22] LE DT, DURHAM JN, SMITH KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade[J]. Science, 2017, 357( 6349): 409- 413. DOI: 10.1126/science.aan6733. [23] HOSSEN MM, MA YM, YIN ZH, et al. Current understanding of CTLA-4: From mechanism to autoimmune diseases[J]. Front Immunol, 2023, 14: 1198365. DOI: 10.3389/fimmu.2023.1198365. [24] LARKIN J, CHIARION-SILENI V, GONZALEZ R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma[J]. N Engl J Med, 2015, 373( 1): 23- 34. DOI: 10.1056/NEJMoa1504030. [25] NI R, HU ZM, TAO R. Advances of immune-checkpoint inhibition of CTLA-4 in pancreatic cancer[J]. Biomed Pharmacother, 2024, 179: 117430. DOI: 10.1016/j.biopha.2024.117430. [26] WOLCHOK JD, CHIARION-SILENI V, GONZALEZ R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma[J]. J Clin Oncol, 2022, 40( 2): 127- 137. DOI: 10.1200/JCO.21.02229. [27] LARKIN J, CHIARION-SILENI V, GONZALEZ R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma[J]. N Engl J Med, 2019, 381( 16): 1535- 1546. DOI: 10.1056/NEJMoa1910836. [28] MOTZER RJ, TANNIR NM, MCDERMOTT DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma[J]. N Engl J Med, 2018, 378( 14): 1277- 1290. DOI: 10.1056/NEJMoa1712126. [29] MOTZER RJ, MCDERMOTT DF, ESCUDIER B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma[J]. Cancer, 2022, 128( 11): 2085- 2097. DOI: 10.1002/cncr.34180. [30] OVERMAN MJ, MCDERMOTT R, LEACH JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer(CheckMate 142): An open-label, multicentre, phase 2 study[J]. Lancet Oncol, 2017, 18( 9): 1182- 1191. DOI: 10.1016/S1470-2045(17)30422-9. [31] OVERMAN MJ, LONARDI S, WONG KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer[J]. J Clin Oncol, 2018, 36( 8): 773- 779. DOI: 10.1200/JCO.2017.76.9901. [32] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma(CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389( 10088): 2492- 2502. DOI: 10.1016/S0140-6736(17)31046-2. [33] BAAS P, SCHERPEREEL A, NOWAK AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma(CheckMate 743): A multicentre, randomised, open-label, phase 3 trial[J]. Lancet, 2021, 397( 10272): 375- 386. DOI: 10.1016/S0140-6736(20)32714-8. [34] DOKI Y, AJANI JA, KATO K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma[J]. N Engl J Med, 2022, 386( 5): 449- 462. DOI: 10.1056/NEJMoa2111380. [35] KELLEY RK, SANGRO B, HARRIS W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study[J]. J Clin Oncol, 2021, 39( 27): 2991- 3001. DOI: 10.1200/JCO.20.03555. [36] CALLAHAN M, AMIN A, KAYE FJ, et al. Nivolumab monotherapy or combination with ipilimumab with or without cobimetinib in previously treated patients with pancreatic adenocarcinoma(CheckMate 032)[J]. J Immunother Cancer, 2024, 12( 2): e007883. DOI: 10.1136/jitc-2023-007883. [37] O'REILLY EM, OH DY, DHANI N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial[J]. JAMA Oncol, 2019, 5( 10): 1431- 1438. DOI: 10.1001/jamaoncol.2019.1588. [38] RENOUF DJ, LOREE JM, KNOX JJ, et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma[J]. Nat Commun, 2022, 13( 1): 5020. DOI: 10.1038/s41467-022-32591-8. [39] LONG B, ZHOU HN, YU ZY, et al. Neoadjuvant cadonilimab plus FLOT chemotherapy in locally advanced gastric/gastroesophageal junction adenocarcinoma: A multicenter, phase 2 study[J]. Med, 2025, 6( 3): 100531. DOI: 10.1016/j.medj.2024.10.008. [40] SHEN L, ZHANG YQ, LI ZY, et al. First-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma: A randomized, double-blind, phase 3 trial[J]. Nat Med, 2025, 31( 4): 1163- 1170. DOI: 10.1038/s41591-024-03450-4. [41] MUNN DH, MELLOR AL. IDO in the tumor microenvironment: Inflammation, counter-regulation, and tolerance[J]. Trends Immunol, 2016, 37( 3): 193- 207. DOI: 10.1016/j.it.2016.01.002. [42] HE X, HE GC, CHU ZX, et al. Discovery of the first potent IDO1/IDO2 dual inhibitors: A promising strategy for cancer immunotherapy[J]. J Med Chem, 2021, 64( 24): 17950- 17968. DOI: 10.1021/acs.jmedchem.1c01305. [43] LI TL, XU D, RUAN Z, et al. Metabolism/immunity dual-regulation thermogels potentiating immunotherapy of glioblastoma through lactate-excretion inhibition and PD-1/PD-L1 blockade[J]. Adv Sci, 2024, 11( 18): 2310163. DOI: 10.1002/advs.202310163. [44] LIANG H, ZHAN JN, CHEN YQ, et al. Tryptophan deficiency induced by indoleamine 2, 3-dioxygenase 1 results in glucose transporter 1-dependent promotion of aerobic glycolysis in pancreatic cancer[J]. MedComm, 2024, 5( 5): e555. DOI: 10.1002/mco2.555. [45] LONG GV, DUMMER R, HAMID O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma(ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study[J]. Lancet Oncol, 2019, 20( 8): 1083- 1097. DOI: 10.1016/S1470-2045(19)30274-8. [46] HELLMANN MD, PAZ-ARES L, BERNABE CARO R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer[J]. N Engl J Med, 2019, 381( 21): 2020- 2031. DOI: 10.1056/NEJMoa1910231. [47] PAZ-ARES L, CIULEANU TE, COBO M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer(CheckMate 9LA): An international, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2021, 22( 2): 198- 211. DOI: 10.1016/S1470-2045(20)30641-0. [48] GAO XY, XU N, LI ZY, et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours(COMPASSION-03): A multicentre, open-label, phase 1b/2 trial[J]. Lancet Oncol, 2023, 24( 10): 1134- 1146. DOI: 10.1016/S1470-2045(23)00411-4. -



 PDF下载 ( 676 KB)
PDF下载 ( 676 KB)

 下载:
下载:


