自身免疫性肝病基于肠道菌群的靶向治疗最新进展
DOI: 10.12449/JCH251226
Latest research advances in intestinal flora-based targeted therapy for autoimmune liver disease
-
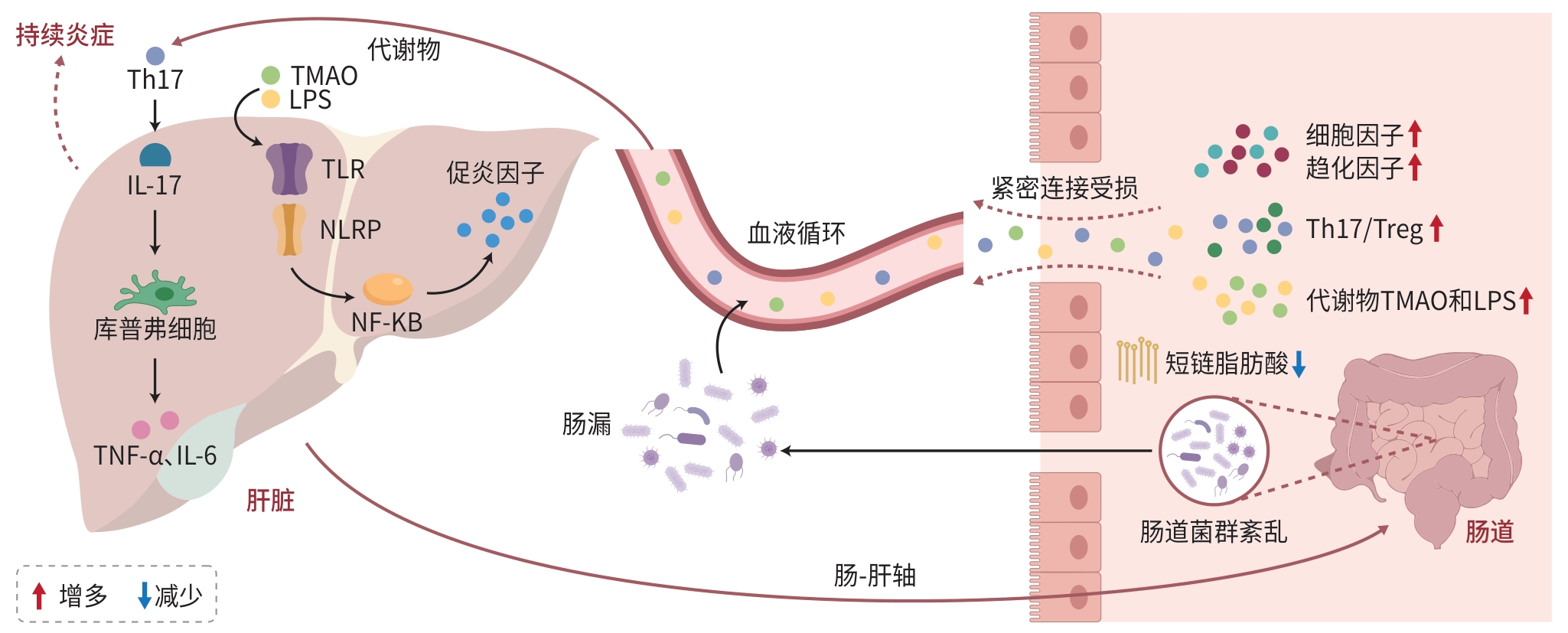
摘要: 自身免疫性肝病(AILD)的发病机制尚未完全阐明,导致其临床疗效存在显著个体差异。近年来的研究表明,肠道菌群及其代谢物通过“肠-肝轴”与AILD发生发展和发病机制之间存在密切关联,基于肠道菌群的靶向治疗方法为治疗AILD患者提供新的策略。本文系统综述了肠道菌群在AILD中的潜在致病机制及靶向治疗策略的最新研究进展,通过解析菌群失衡介导免疫紊乱的分子机制,探讨靶向调控肠道微生态实现治疗的可能性,以期为临床诊疗提供理论依据和转化方向。Abstract: The pathogenesis of autoimmune liver disease (AILD) remains unclear, leading to significant individual differences in clinical outcomes. Recent studies have shown that intestinal flora and its metabolites are closely associated with the development, progression, and pathogenesis of AILD through the gut-liver axis, and targeted therapy based on intestinal flora has provided new strategies for the treatment of AILD. This article systematically reviews the potential pathogenic mechanism of intestinal flora in AILD and the latest research advances in targeted therapeutic strategies and explores the possibility of targeted regulation of intestinal microecology to achieve treatment by analyzing the molecular mechanisms of immune disorders mediated by imbalance of intestinal flora, in order to provide a theoretical basis for clinical diagnosis and treatment.
-
Key words:
- Autoimmune Liver Disease /
- Gastrointestinal Microbiome /
- Targeted Therapy
-
表 1 靶向肠道菌群策略优缺点及临床研究总结
Table 1. Summary of advantages and disadvantages of targeted gut flora strategies and clinical studies
治疗方式 核心优势 主要局限 样本量(例) 疗效 局限性 抗生素 快速杀菌、控制感染 菌群失调,易产生耐
药性50~100 短期有效,炎症指标显著
下降不具备物种特异性,耐药
性高FMT 快速重建菌群,改善肠道
环境感染风险,操作复杂 <50 肠道菌群多样性增加,症
状迅速缓解缺少大型验证,长期效果
需观察益生菌 安全性高,辅助调节菌群 疗效有限,菌株依赖
性强50~100 轻中症患者改善,免疫指
标好转重症患者疗效差 噬菌体 精准杀菌,耐药性小 研发周期长,技术难
度大未开展 未知 技术要求高,特异性高 中药 多机制调节,副作用小 证据不足,成分复杂 100~150 症状和肝功能指标改善,
副作用少成分复杂,机制复杂 性激素 快速调节免疫反应 内分泌紊乱、血栓风
险增加<50 炎症缓解,免疫调节作用 内分泌相关副作用,范围
局限 -
[1] QIU ZX, HUANG LX, WANG XX, et al. Exploring the pathogenesis of autoimmune liver diseases from the heterogeneity of target cells[J]. J Clin Transl Hepatol, 2024, 12( 7): 659- 666. DOI: 10.14218/JCTH.2023.00531. [2] ZHAO YJ, XIE L, ZHANG YT, et al. Pyroptosis: A new bridge connecting gut microbiota and liver diseases[J]. J Clin Hepatol, 2024, 40( 9): 1908- 1915. DOI: 10.12449/JCH240930.赵奕杰, 谢露, 张亚亭, 等. 细胞焦亡: 连接肠道菌群与肝脏疾病的新桥梁[J]. 临床肝胆病杂志, 2024, 40( 9): 1908- 1915. DOI: 10.12449/JCH240930. [3] LI R, MAO ZS, YE XJ, et al. Human gut microbiome and liver diseases: From correlation to causation[J]. Microorganisms, 2021, 9( 5): 1017. DOI: 10.3390/microorganisms9051017. [4] CHENG ZL, YANG L, CHU HK. The gut microbiota: A novel player in autoimmune hepatitis[J]. Front Cell Infect Microbiol, 2022, 12: 947382. DOI: 10.3389/fcimb.2022.947382. [5] BERINGER A, MIOSSEC P. IL-17 and IL-17-producing cells and liver diseases, with focus on autoimmune liver diseases[J]. Autoimmun Rev, 2018, 17( 12): 1176- 1185. DOI: 10.1016/j.autrev.2018.06.008. [6] YANG Y, CHOI J, CHEN Y, et al. E. coli and the etiology of human PBC: Antimitochondrial antibodies and spreading determinants[J]. Hepatology, 2022, 75( 2): 266- 279. DOI: 10.1002/hep.32172. [7] TERJUNG B, SPENGLER U. Atypical p-ANCA in PSC and AIH: A Hint Toward a“leaky gut”[J]. Clin Rev Allergy Immunol, 2009, 36( 1): 40- 51. DOI: 10.1007/s12016-008-8088-8. [8] GOEL R, EAPEN CE. Recognizing dysfunctional innate and adaptive immune responses contributing to liver damage in patients with cirrhosis[J]. J Clin Exp Hepatol, 2022, 12( 3): 993- 1002. DOI: 10.1016/j.jceh.2021.10.001. [9] LIU H, ZHAO J, ZHANG WJ, et al. Impacts of sodium butyrate on intestinal mucosal barrier and intestinal microbial community in a weaned piglet model[J]. Front Microbiol, 2022, 13: 1041885. DOI: 10.3389/fmicb.2022.1041885. [10] KAYAMA H, OKUMURA R, TAKEDA K. Interaction between the microbiota, epithelia, and immune cells in the intestine[J]. Annu Rev Immunol, 2020, 38: 23- 48. DOI: 10.1146/annurev-immunol-070119-115104. [11] INAMINE T, SCHNABL B. Immunoglobulin A and liver diseases[J]. J Gastroenterol, 2018, 53( 6): 691- 700. DOI: 10.1007/s00535-017-1400-8. [12] LAPIDOT Y, AMIR A, BEN-SIMON S, et al. Alterations of the salivary and fecal microbiome in patients with primary sclerosing cholangitis[J]. Hepatol Int, 2021, 15( 1): 191- 201. DOI: 10.1007/s12072-020-10089-z. [13] ÖZDIRIK B, SCHERF M, BRUMERCEK A, et al. Biliary microbial patterns in primary sclerosing cholangitis are linked to poorer transplant-free survival[J]. Hepatol Commun, 2023, 7( 6): e0156. DOI: 10.1097/HC9.000-0000000000156. [14] BABU G, MOHANTY B. Neurotensin modulation of lipopolysaccharide induced inflammation of gut-liver axis: Evaluation using neurotensin receptor agonist and antagonist[J]. Neuropeptides, 2023, 97: 102297. DOI: 10.1016/j.npep.2022.102297. [15] VERMA S, REDDY P, SOWDHAMINI R. Integrated approaches for the recognition of small molecule inhibitors for Toll-like receptor 4[J]. Comput Struct Biotechnol J, 2023, 21: 3680- 3689. DOI: 10.1016/j.csbj.2023.07.026. [16] KIM YS, HURLEY EH, PARK Y, et al. Primary sclerosing cholangitis(PSC) and inflammatory bowel disease(IBD): A condition exemplifying the crosstalk of the gut-liver axis[J]. Exp Mol Med, 2023, 55( 7): 1380- 1387. DOI: 10.1038/s12276-023-01042-9. [17] KUMMEN M, THINGHOLM LB, RÜHLEMANN MC, et al. Altered gut microbial metabolism of essential nutrients in primary sclerosing cholangitis[J]. Gastroenterology, 2021, 160( 5): 1784- 1798. e 0. DOI: 10.1053/j.gastro.2020.12.058. [18] KOTLYAROV S. Importance of the gut microbiota in the gut-liver axis in normal and liver disease[J]. World J Hepatol, 2024, 16( 6): 878- 882. DOI: 10.4254/wjh.v16.i6.878. [19] BIAGIOLI M, CARINO A, FIORUCCI C, et al. GPBAR1 functions as gatekeeper for liver NKT cells and provides counterregulatory signals in mouse models of immune-mediated hepatitis[J]. Cell Mol Gastroenterol Hepatol, 2019, 8( 3): 447- 473. DOI: 10.1016/j.jcmgh.2019.06.003. [20] PAIK D, YAO LN, ZHANG YC, et al. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites[J]. Nature, 2022, 603( 7903): 907- 912. DOI: 10.1038/s41586-022-04480-z. [21] ZHANG L, YANG L, CHU HK. Targeting gut microbiota for the treatment of primary biliary cholangitis: From bench to bedside[J]. J Clin Transl Hepatol, 2023, 11( 4): 958- 966. DOI: 10.14218/JCTH.2022.00408. [22] LIWINSKI T, ZENOUZI R, JOHN C, et al. Alterations of the bile microbiome in primary sclerosing cholangitis[J]. Gut, 2020, 69( 4): 665- 672. DOI: 10.1136/gutjnl-2019-318416. [23] JIANG BR, YUAN GH, WU JL, et al. Prevotella copri ameliorates cholestasis and liver fibrosis in primary sclerosing cholangitis by enhancing the FXR signalling pathway[J]. Biochim Biophys Acta Mol Basis Dis, 2022, 1868( 3): 166320. DOI: 10.1016/j.bbadis.2021.166320. [24] RAHIMPOUR S, NASIRI-TOOSI M, KHALILI H, et al. A triple blinded, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of oral vancomycin in primary sclerosing cholangitis: A pilot study[J]. J Gastrointestin Liver Dis, 2016, 25( 4): 457- 464. DOI: 10.15403/jgld.2014.1121.254.rah. [25] BRITTO SL, HOFFMAN KL, TESSIER ME, et al. Microbiome responses to vancomycin treatment in a child with primary sclerosing cholangitis and ulcerative colitis[J]. ACG Case Rep J, 2021, 8( 5): e00577. DOI: 10.14309/crj.0000000000000577. [26] FÄRKKILÄ M, KARVONEN AL, NURMI H, et al. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: A randomized placebo-controlled trial[J]. Hepatology, 2004, 40( 6): 1379- 1386. DOI: 10.1002/hep.20457. [27] di GIORGIO A, TULONE A, NICASTRO E, et al. Use of oral vancomycin in children with autoimmune liver disease: A single centre experience[J]. World J Hepatol, 2021, 13( 12): 2113- 2127. DOI: 10.4254/wjh.v13.i12.2113. [28] LI LP, KANG YB. The gut microbiome and autoimmune hepatitis: Implications for early diagnostic biomarkers and novel therapies[J]. Mol Nutr Food Res, 2023, 67( 24): e2300043. DOI: 10.1002/mnfr.20-2300043. [29] PHILIPS CA, AUGUSTINE P, PHADKE N. Healthy donor fecal microbiota transplantation for recurrent bacterial cholangitis in primary sclerosing cholangitis- a single case report[J]. J Clin Transl Hepatol, 2018, 6( 4): 438- 441. DOI: 10.14218/JCTH.2018.00033. [30] ALLEGRETTI JR, KASSAM Z, CARRELLAS M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: A pilot clinical trial[J]. Am J Gastroenterol, 2019, 114( 7): 1071- 1079. DOI: 10.14309/ajg.0000000000000115. [31] JIA W, LI YT, CHEUNG KCP, et al. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis[J]. Sci China Life Sci, 2024, 67( 5): 865- 878. DOI: 10.1007/s11427-023-2353-0. [32] ZHANG HX, LIU M, LIU X, et al. Bifidobacterium animalis ssp. lactis 420 mitigates autoimmune hepatitis through regulating intestinal barrier and liver immune cells[J]. Front Immunol, 2020, 11: 569104. DOI: 10.3389/fimmu.2020.569104. [33] MA L, ZHANG LW, ZHUANG Y, et al. Lactobacillus improves the effects of prednisone on autoimmune hepatitis via gut microbiota-mediated follicular helper T cells[J]. Cell Commun Signal, 2022, 20( 1): 83. DOI: 10.1186/s12964-021-00819-7. [34] KANG YB, KUANG XY, YAN H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway[J]. mSystems, 2023, 8( 2). DOI: 10.1128/msystems.01127-22. [35] RODRÍGUEZ-PASTÉN A, PÉREZ-HERNÁNDEZ N, AÑORVE-MORGA J, et al. The activity of prebiotics and probiotics in hepatogastrointestinal disorders and diseases associated with metabolic syndrome[J]. Int J Mol Sci, 2022, 23( 13): 7229. DOI: 10.3390/ijms23137229. [36] BOGATIC D, BRYANT RV, LYNCH KD, et al. Systematic review: Microbial manipulation as therapy for primary sclerosing cholangitis[J]. Aliment Pharmacol Ther, 2023, 57( 1): 23- 36. DOI: 10.1111/apt.17251. [37] LI C, NIU ZH, ZOU MJ, et al. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms[J]. J Dairy Sci, 2020, 103( 7): 5816- 5829. DOI: 10.3168/jds.2019-18003. [38] HUANG GF, YANG JH. Relationship between autoantibodies and biochemical responses to different doses of ursodeoxycholic acid for the treatment of primary biliary cholangitis[J]. Med J Chin People’s Liberat Army, 2022, 47( 2): 143- 149. DOI: 10.11855/j.issn.0577-7402.2022.02.0143.黄桂芳, 杨晋辉. 自身抗体与熊去氧胆酸治疗原发性胆汁性胆管炎生化应答的关系探讨[J]. 解放军医学杂志, 2022, 47( 2): 143- 149. DOI: 10.11855/j.issn.0577-7402.2022.02.0143. [39] Chinese Society of Hepatology, Chinese Medical Association. Guideline on the management of cholestasis liver diseases(2021)[J]. J Clin Hepatol, 2022, 38( 1): 62- 69. DOI: 10.3969/j.issn.1001-5256.2022.01.010.中华医学会肝病学分会. 胆汁淤积性肝病管理指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 62- 69. DOI: 10.3969/j.issn.1001-5256.2022.01.010. [40] WU JW, LYU SX, GUO D, et al. Protective effects of YCHD on the autoimmune hepatitis mice model induced by Ad-CYP2D6 through modulating the Th1/Treg ratio and intestinal flora[J]. Front Immunol, 2024, 15: 1488125. DOI: 10.3389/fimmu.2024.1488125. [41] ZHOU XH, GONG DY, ZHONG S. Protective effect of modified Yinchenhao decoction against acute liver injury in rats by inhibiting TLR4 signaling pathway[J]. J Shanxi Med Univ, 2021, 52( 9): 1143- 1148. DOI: 10.13753/j.issn.1007-6611.2021.09.009.周兴华, 龚道银, 钟森. 加味茵陈蒿汤通过抑制TLR4信号通路对大鼠急性肝损伤的保护作用[J]. 山西医科大学学报, 2021, 52( 9): 1143- 1148. DOI: 10.13753/j.issn.1007-6611.2021.09.009. [42] YANG H, LIU QQ, LIU HX, et al. Berberine alleviates concanavalin A-induced autoimmune hepatitis in mice by modulating the gut microbiota[J]. Hepatol Commun, 2024, 8( 4): e0381. DOI: 10.1097/HC9.0000000000000381. [43] ZENG X, LIU MH, XIONG Y, et al. Pien Tze Huang alleviates Concanavalin A-induced autoimmune hepatitis by regulating intestinal microbiota and memory regulatory T cells[J]. World J Gastroenterol, 2023, 29( 45): 5988- 6016. DOI: 10.3748/wjg.v29.i45.5988. [44] Expert Committee on Hepatology, Doctor Society of Integrative Medicine, Chinese Medical Doctor Association. Experts consensus on integrated traditional Chinese and Western medicine diagnosis and treatment of primary biliary cholangitis[J]. J Clin Hepatol, 2024, 40( 9): 1757- 1766. DOI: 10.12449/JCH240907.中国医师协会中西医结合医师分会肝病学专家委员会. 原发性胆汁性胆管炎中西医结合诊疗专家共识[J]. 临床肝胆病杂志, 2024, 40( 9): 1757- 1766. DOI: 10.12449/JCH240907. [45] GÓRSKI A, JOŃCZYK-MATYSIAK E, ŁUSIAK-SZELACHOWSKA M, et al. Therapeutic potential of phages in autoimmune liver diseases[J]. Clin Exp Immunol, 2018, 192( 1): 1- 6. DOI: 10.1111/cei.13092. [46] NIE QX, LUO X, WANG K, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway[J]. Cell, 2024, 187( 11): 2717- 2734. e 33. DOI: 10.1016/j.cell.2024.03.034. [47] FUJIKI J, SCHNABL B. Phage therapy: Targeting intestinal bacterial microbiota for the treatment of liver diseases[J]. JHEP Rep, 2023, 5( 12): 100909. DOI: 10.1016/j.jhepr.2023.100909. [48] WU ZH, SUN Y, HUANG WB, et al. Direct and indirect effects of estrogens, androgens and intestinal microbiota on colorectal cancer[J]. Front Cell Infect Microbiol, 2024, 14: 1458033. DOI: 10.3389/fcimb.2024.1458033. [49] ZHANG HX, LIU M, LIU X, et al. Bifidobacterium animalis ssp. lactis 420 mitigates autoimmune hepatitis through regulating intestinal barrier and liver immune cells[J]. Front Immunol, 2020, 11: 569104. DOI: 10.3389/fimmu.2020.569104. [50] ZU Y, YANG JY, ZHANG CL, et al. The pathological mechanisms of estrogen-induced cholestasis: Current perspectives[J]. Front Pharmacol, 2021, 12: 761255. DOI: 10.3389/fphar.2021.761255. -



 PDF下载 ( 785 KB)
PDF下载 ( 785 KB)

 下载:
下载:


