代谢相关脂肪性肝病肝纤维化的代谢重编程机制
DOI: 10.12449/JCH251225
The metabolic reprogramming mechanisms of liver fibrosis in metabolic associated fatty liver disease
-
摘要: 代谢相关脂肪性肝病(MAFLD)肝纤维化的发病率逐年上升,其持续进展最终可导致肝硬化甚至肝癌。糖代谢、脂代谢及蛋白质代谢的途径发生改变并为肝星状细胞(HSC)的活化提供必要的能量支持,从而促进肝纤维化进展。现有研究阐明了上述代谢重编程在调控HSC活化中的具体作用机制,如HSC活化与糖酵解增强、乳酸微环境形成、脂肪酸β氧化抑制、脂毒性累积、谷氨酰胺分解增强以及蛋白质稳定性和S-腺苷蛋氨酸(SAM)水平变化等,且乳酸微环境形成、脂毒性累积以及SAM水平等变化可加速肝纤维化进展。本文系统综述了MAFLD肝纤维化中代谢途径的变化以及相互间的协同作用,以期为揭示该病的发病机制和开发新型治疗策略提供重要理论依据。Abstract: There has been a gradual increase in the incidence rate of metabolic associated fatty liver disease (MAFLD)-related liver fibrosis year by year, and its progression may eventually lead to liver cirrhosis and even liver cancer. There are changes in the pathways of glucose metabolism, lipid metabolism, and protein metabolism, which provide necessary energy support for the activation of hepatic stellate cells (HSC), thereby promoting the progression of liver fibrosis. Existing studies have clarified the specific mechanisms of metabolic reprogramming in regulating the activation of HSC such as the activation of HSC and enhanced glycolysis, the formation of a lactic acid microenvironment, the inhibition of fatty acid β-oxidation, the accumulation of lipotoxicity, enhanced glutamine decomposition, and changes in protein stability and S-adenosylmethionine (SAM). Moreover, the formation of a lactic acid microenvironment, the accumulation of lipotoxicity, and changes in SAM can accelerate the progression of liver fibrosis. This article systematically reviews the changes in related metabolic pathways in MAFLD-related liver fibrosis and the synergistic effect between them, in order to provide an important theoretical basis for revealing the pathogenesis of this disease and developing new treatment strategies.
-
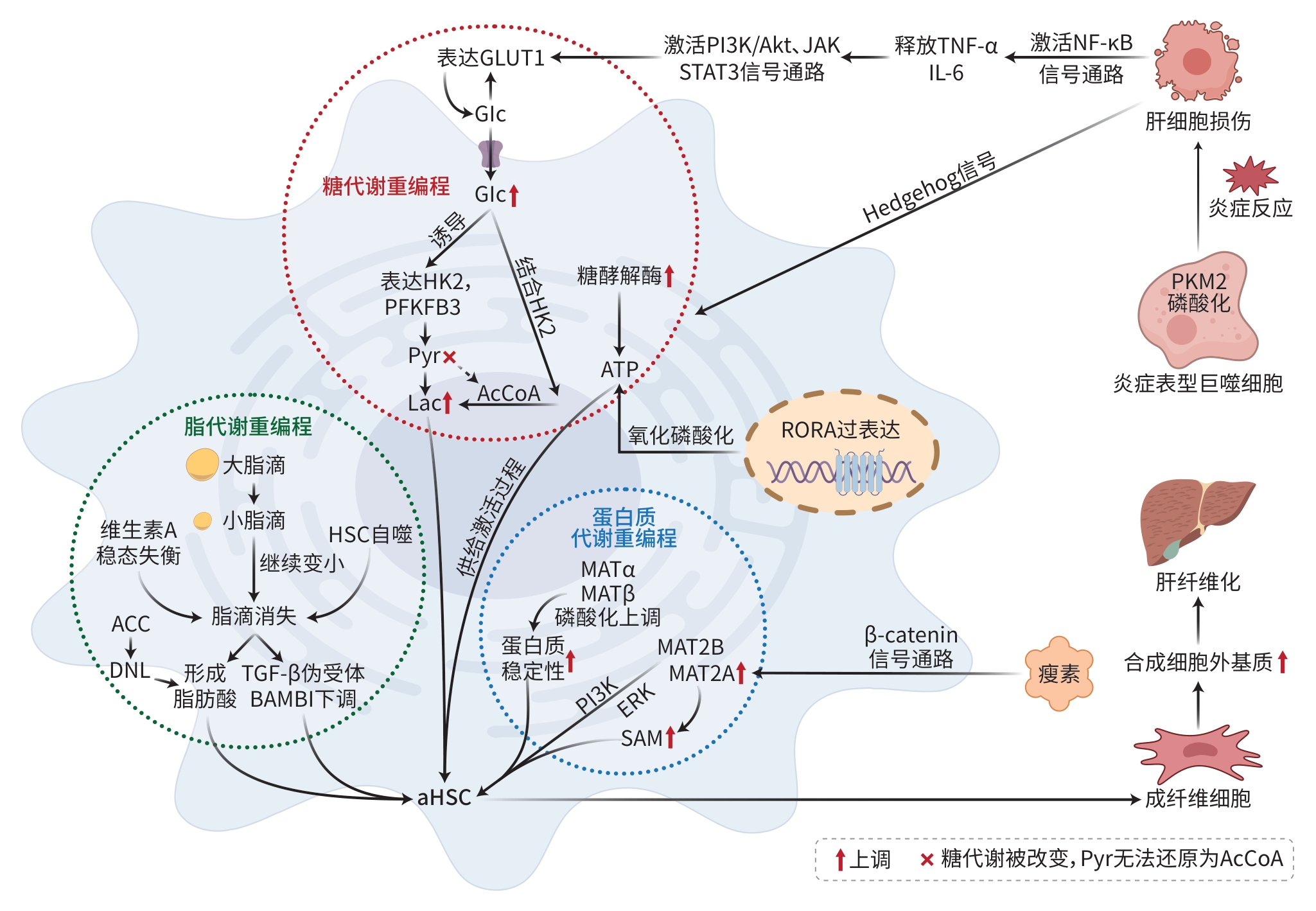
注: GLUT1,葡萄糖转运蛋白1;Glc,葡萄糖;HK2,己糖激酶2;PFKFB3,果糖2,6双磷酸酶3;Pyr,丙酮酸;Lac,乳酸;AcCoA,乙酰辅酶A;ACC,乙酰辅酶A羧化酶;ATP,三磷酸腺苷;DNL,从头脂肪生成;BAMBI,BMP和激活素膜结合抑制剂;aHSC,活化的HSC;RORA,维甲酸相关孤儿受体α;PKM2,丙酮酸激酶M2。
图 1 MAFLD肝纤维化的代谢重编程机制
Figure 1. Metabolic reprogramming mechanism of liver fibrosis in metabolic associated fatty liver disease
-
[1] Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of metabolic dysfunction-associated related(non-alcoholic) fatty liver disease(Version 2024)[J]. J Pract Hepatol, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501-113-20240327-00163.中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 实用肝脏病杂志, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163. [2] RONG L, ZOU JY, RAN W, et al. Advancements in the treatment of non-alcoholic fatty liver disease(NAFLD)[J]. Front Endocrinol, 2023, 13: 1087260. DOI: 10.3389/fendo.2022.1087260. [3] LI XH, FAN JG. Controversy over renaming nonalcoholic fatty liver disease as metabolic fatty liver disease[J]. Chin Hepatol, 2023, 28( 5): 505- 507. DOI: 10.14000/j.cnki.issn.1008-1704.2023.05.001.李旭辉, 范建高. 非酒精性脂肪性肝病更名代谢相关脂肪性肝病的争议[J]. 肝脏, 2023, 28( 5): 505- 507. DOI: 10.14000/j.cnki.issn.1008-1704.2023.05.001. [4] KANWAL F, NEUSCHWANDER-TETRI BA, LOOMBA R, et al. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease[J]. Hepatology, 2024, 79( 5): 1212- 1219. DOI: 10.1097/HEP.000000000-0000670. [5] RAMÍREZ-MEJÍA MM, QI XS, ABENAVOLI L, et al. Metabolic dysfunction: The silenced connection with fatty liver disease[J]. Ann Hepatol, 2023, 28( 6): 101138. DOI: 10.1016/j.aohep.2023.101138. [6] LU R, LIU Y, HONG TP. Epidemiological characteristics and management of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in China: A narrative review[J]. Diabetes Obes Metab, 2023, 25( Suppl 1): 13- 26. DOI: 10.1111/dom.15014. [7] FOUDA S, PAPPACHAN JM. Metabolic-associated fatty liver disease: A disastrous human health challenge[J]. Endocrinol Metab Clin N Am, 2023, 52( 3): xv-xvi. DOI: 10.1016/j.ecl.2023.03.001. [8] CHO JY, SOHN W. The growing burden of non-alcoholic fatty liver disease on mortality[J]. Clin Mol Hepatol, 2023, 29( 2): 374- 376. DOI: 10.3350/cmh.2023.0084. [9] DU K, HYUN J, PREMONT RT, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells[J]. Gastroenterology, 2018, 154( 5): 1465- 1479.e13. DOI: 10.1053/j.gastro.2017.12.022. [10] CHEN J, XU L, CAO ZM, et al. Research progress on cell and molecular mechanism of traditional Chinese medicine against liver fibrosis[J]. China J Tradit Chin Med Pharm, 2022, 37( 3): 1564- 1569.陈静, 徐蕾, 曹正民, 等. 中医药抗肝纤维化细胞分子机制研究进展[J]. 中华中医药杂志, 2022, 37( 3): 1564- 1569. [11] ALISI A, MCCAUGHAN G, GRØNBÆK H. Role of gut microbiota and immune cells in metabolic-associated fatty liver disease: Clinical impact[J]. Hepatol Int, 2024, 18( Suppl 2): 861- 872. DOI: 10.1007/s12072-024-10674-6. [12] ZHOU RN, WANG Y, HU JH, et al. Effect of total flavonoids from Astragali Complanati Semen on improving hepatic fibrosis in mice by regulating hepatic stellate cells iron metabolism[J]. Chin Tradit Herb Drugs, 2025, 56( 9): 3131- 3139. DOI: 10.7501/j.issn.0253-2670.2025.09.011.周瑞娜, 王洋, 胡锦航, 等. 沙苑子总黄酮通过调节肝星状细胞铁代谢改善小鼠肝纤维化[J]. 中草药, 2025, 56( 9): 3131- 3139. DOI: 10.7501/j.issn.0253-2670.2025.09.011. [13] LIANG C, LIU JJ, JIANG MX, et al. The advancement of targeted regulation of hepatic stellate cells using traditional Chinese medicine for the treatment of liver fibrosis[J]. J Ethnopharmacol, 2025, 341: 119298. DOI: 10.1016/j.jep.2024.119298. [14] MEJIAS M, GALLEGO J, NARANJO-SUAREZ S, et al. CPEB4 increases expression of PFKFB3 to induce glycolysis and activate mouse and human hepatic stellate cells, promoting liver fibrosis[J]. Gastroenterology, 2020, 159( 1): 273- 288. DOI: 10.1053/j.gastro.2020.03.008. [15] WANG S, LI K, PICKHOLZ E, et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis[J]. Sci Transl Med, 2023, 15( 677): eadd3949. DOI: 10.1126/scitranslmed.add3949. [16] ALVES-BEZERRA M, COHEN DE. Triglyceride metabolism in the liver[J]. Compr Physiol, 2017, 8( 1): 1- 8. DOI: 10.1002/cphy.c170012. [17] LIU Y, WEN B, HE CY, et al. Research progress on regulation of hepatic macrophages on the activation of hepatic stellate cells in liver fibrosis[J]. World Sci Technol Mod Tradit Chin Med, 2022, 24( 3): 1097- 1102. DOI: 10.11842/wst.20210704008.刘洋, 文彬, 何春雨, 等. 肝巨噬细胞调控肝星状细胞活化影响肝纤维化的研究进展[J]. 世界科学技术-中医药现代化, 2022, 24( 3): 1097- 1102. DOI: 10.11842/wst.20210704008. [18] TRIVEDI P, WANG S, FRIEDMAN SL. The power of plasticity-metabolic regulation of hepatic stellate cells[J]. Cell Metab, 2021, 33( 2): 242- 257. DOI: 10.1016/j.cmet.2020.10.026. [19] YANG F, HILAKIVI-CLARKE L, SHAHA A, et al. Metabolic reprogramming and its clinical implication for liver cancer[J]. Hepatology, 2023, 78( 5): 1602- 1624. DOI: 10.1097/HEP.0000000000000005. [20] QU HD, LIU JL, ZHANG D, et al. Glycolysis in chronic liver diseases: Mechanistic insights and therapeutic opportunities[J]. Cells, 2023, 12( 15): 1930. DOI: 10.3390/cells12151930. [21] HAO MM, LIU L, YI LP, et al. Research progress on the mechanism of regulating glycolysis of hepatic stellate cells against liver fibrosis and the prevention and treatment of traditional Chinese medicine[J]. Tradit Chin Drug Res Clin Pharmacol, 2024, 35( 7): 1101- 1106. DOI: 10.19378/j.issn.1003-9783.2024.07.019.郝梦梦, 刘璐, 易浪平, 等. 调控肝星状细胞糖酵解抗肝纤维化的机制及中医药防治研究进展[J]. 中药新药与临床药理, 2024, 35( 7): 1101- 1106. DOI: 10.19378/j.issn.1003-9783.2024.07.019. [22] WU SH, HE YH, LI JX, et al. Overview of the role of metabolic reprogramming of hepatic stellate cells in liver fibrosis[J]. J Guangdong Med Coll, 2024, 42( 5): 518- 522, 534. DOI: 10.3969/j.issn.1005-4057.2024.05.013.武士豪, 何钰宏, 李嘉兴, 等. 肝星状细胞的代谢重编程在肝纤维化发展中的研究现状[J]. 广东医科大学学报, 2024, 42( 5): 518- 522, 534. DOI: 10.3969/j.issn.1005-4057.2024.05.013. [23] ZHOU YQ, YAN JX, HUANG H, et al. The m(6)a reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis[J]. Cell Death Dis, 2024, 15( 3): 189. DOI: 10.1038/s41419-024-06509-9. [24] LEE NCW, CARELLA MA, PAPA S, et al. High expression of glycolytic genes in cirrhosis correlates with the risk of developing liver cancer[J]. Front Cell Dev Biol, 2018, 6: 138. DOI: 10.3389/fcell.2018.00138. [25] RHO H, TERRY AR, CHRONIS C, et al. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis[J]. Cell Metab, 2023, 35( 8): 1406- 1423.e8. DOI: 10.1016/j.cmet.2023.06.013. [26] DU DY, LIU C, QIN MY, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma[J]. Acta Pharm Sin B, 2022, 12( 2): 558- 580. DOI: 10.1016/j.apsb.2021.09.019. [27] HORN P, TACKE F. Metabolic reprogramming in liver fibrosis[J]. Cell Metab, 2024, 36( 7): 1439- 1455. DOI: 10.1016/j.cmet.2024.05.003. [28] WANG XH, WANG YG, BAI B, et al. PKMζ, a brain-specific PKCζ isoform, is required for glycolysis and myofibroblastic activation of hepatic stellate cells[J]. Cell Mol Gastroenterol Hepatol, 2025, 19( 3): 101429. DOI: 10.1016/j.jcmgh.2024.101429. [29] LI LS, LEI Q, ZHEN YF, et al. Lactate dehydrogenase inhibition protects against hepatic fibrosis by regulating metabolic reprogramming of hepatic stellate cells[J]. J Agric Food Chem, 2024, 72( 50): 27953- 27964. DOI: 10.1021/acs.jafc.4c08211. [30] MARTÍNEZ GARCÍA de la TORRE RA, VALLVERDÚ J, XU ZQ, et al. Trajectory analysis of hepatic stellate cell differentiation reveals metabolic regulation of cell commitment and fibrosis[J]. Nat Commun, 2025, 16( 1): 1489. DOI: 10.1038/s41467-025-56024-4. [31] EZHILARASAN D. Mitochondria: A critical hub for hepatic stellate cells activation during chronic liver diseases[J]. Hepatobiliary Pancreat Dis Int, 2021, 20( 4): 315- 322. DOI: 10.1016/j.hbpd.2021.04.010. [32] PAN M, LI HY, SHI XY. A new target for hepatic fibrosis prevention and treatment: The Warburg effect[J]. Front Biosci, 2024, 29( 9): 321. DOI: 10.31083/j.fbl2909321. [33] XIAO Y, WU ZN, LU J, et al. Role of metabolic reprogramming-mediated hepatic stellate cell activation in the pathogenesis of hepatic fibrosis[J]. Chin J Hepatol, 2024, 32( 11): 1053- 1056. DOI: 10.3760/cma.j.cn501113-20240223-00088.肖滢, 吴治念, 鲁洁, 等. 代谢重编程介导的肝星状细胞活化在肝纤维化发生机制中的作用[J]. 中华肝脏病杂志, 2024, 32( 11): 1053- 1056. DOI: 10.3760/cma.j.cn501113-20240223-00088. [34] LEE JL, WANG YC, HSU YA, et al. Galectin-12 modulates Kupffer cell polarization to alter the progression of nonalcoholic fatty liver disease[J]. Glycobiology, 2023, 33( 8): 673- 682. DOI: 10.1093/glycob/cwad062. [35] FILALI-MOUNCEF Y, HUNTER C, ROCCIO F, et al. The ménage à trois of autophagy, lipid droplets and liver disease[J]. Autophagy, 2022, 18( 1): 50- 72. DOI: 10.1080/15548627.2021.1895658. [36] YANG T, ZHAO DL, ZHOU YY, et al. Glucose, lipid and protein metabolism of hepatic stellate cells: A novel target against liver fibrosis[J]. Chin Pharmacol Bull, 2021, 37( 7): 902- 905. DOI: 10.3969/j.issn.1001-1978.2021.07.004.杨婷, 赵丹雳, 周媛媛, 等. 肝星状细胞糖脂蛋白质代谢: 抗肝纤维化的新靶标[J]. 中国药理学通报, 2021, 37( 7): 902- 905. DOI: 10.3969/j.issn.1001-1978.2021.07.004. [37] FONDEVILA MF, NOVOA E, GONZALEZ-RELLAN MJ, et al. p63 controls metabolic activation of hepatic stellate cells and fibrosis via an HER2-ACC1 pathway[J]. Cell Rep Med, 2024, 5( 2): 101401. DOI: 10.1016/j.xcrm.2024.101401. [38] BATES J, VIJAYAKUMAR A, GHOSHAL S, et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation[J]. J Hepatol, 2020, 73( 4): 896- 905. DOI: 10.1016/j.jhep.2020.04.037. [39] DELGADO ME, CÁRDENAS BI, FARRAN N, et al. Metabolic reprogramming of liver fibrosis[J]. Cells, 2021, 10( 12): 3604. DOI: 10.3390/cells10123604. [40] HU SW, LI R, GONG DX, et al. Atf3-mediated metabolic reprogramming in hepatic macrophage orchestrates metabolic dysfunction-associated steatohepatitis[J]. Sci Adv, 2024, 10( 30): eado3141. DOI: 10.1126/sciadv.ado3141. [41] SUK FM, HSU FY, LEE YC, et al. Dietary oxidized frying oil activates hepatic stellate cells and accelerates the severity of carbon tetrachloride- and thioacetamide-induced liver fibrosis in mice[J]. J Nutr Biochem, 2023, 115: 109267. DOI: 10.1016/j.jnutbio.2023.109267. [42] YIN XC, PENG J, GU LH, et al. Targeting glutamine metabolism in hepatic stellate cells alleviates liver fibrosis[J]. Cell Death Dis, 2022, 13( 11): 955. DOI: 10.1038/s41419-022-05409-0. [43] LI L, LEI Q, ZHEN Y, et al. Lactate dehydrogenase inhibition protects against hepatic fibrosis by regulating metabolic reprogramming of hepatic stellate cells[J]. J Agric Food Chem, 2024, 72( 50): 27953- 27964. DOI: 10.1021/acs.jafc.4c08211. [44] YING KL, ZENG Y, XU J, et al. LncRNA SNHG11 reprograms glutaminolysis in hepatic stellate cells via Wnt/β-catenin/GLS axis[J]. Biochem Pharmacol, 2024, 221: 116044. DOI: 10.1016/j.bcp.2024.116044. [45] HUANG RH, CUI HY, YAHYA ALI ALSHAMI MA, et al. LOX-1 rewires glutamine ammonia metabolism to drive liver fibrosis[J]. Mol Metab, 2025, 96: 102132. DOI: 10.1016/j.molmet.2025.102132. [46] ZHAO XT, AMEVOR FK, XUE XY, et al. Remodeling the hepatic fibrotic microenvironment with emerging nanotherapeutics: A comprehensive review[J]. J Nanobiotechnol, 2023, 21( 1): 121. DOI: 10.1186/s12951-023-01876-5. [47] YAN YF, ZENG JF, XING LH, et al. Extra- and intra-cellular mechanisms of hepatic stellate cell activation[J]. Biomedicines, 2021, 9( 8): 1014. DOI: 10.3390/biomedicines9081014. [48] DU K, CHITNENI SK, SUZUKI A, et al. Increased glutaminolysis marks active scarring in nonalcoholic steatohepatitis progression[J]. Cell Mol Gastroenterol Hepatol, 2020, 10( 1): 1- 21. DOI: 10.1016/j.jcmgh.2019.12.006. [49] EL-ASHMAWY NE, AL-ASHMAWY GM, FAKHER HE, et al. The role of WNT/β-catenin signaling pathway and glutamine metabolism in the pathogenesis of CCl(4)-induced liver fibrosis: Repositioning of niclosamide and concerns about lithium[J]. Cytokine, 2020, 136: 155250. DOI: 10.1016/j.cyto.2020.155250. [50] LI CZ, GUI G, ZHANG L, et al. Overview of methionine adenosyltransferase 2A(MAT2A) as an anticancer target: Structure, function, and inhibitors[J]. J Med Chem, 2022, 65( 14): 9531- 9547. DOI: 10.1021/acs.jmedchem.2c00395. [51] ALARCÓN-VILA C, INSAUSTI-URKIA N, TORRES S, et al. Dietary and genetic disruption of hepatic methionine metabolism induce acid sphingomyelinase to promote steatohepatitis[J]. Redox Biol, 2023, 59: 102596. DOI: 10.1016/j.redox.2022.102596. [52] RAMANI K, YANG HP, KUHLENKAMP J, et al. Changes in the expression of methionine adenosyltransferase genes and S-adenosylmethio‑nine homeostasis during hepatic stellate cell activation[J]. Hepatology, 2010, 51( 3): 986- 995. DOI: 10.1002/hep.23411. [53] LI ZH, WANG FX, LIANG BY, et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication[J]. Signal Transduct Target Ther, 2020, 5( 1): 280. DOI: 10.1038/s41392-020-00349-7. [54] YANG B, LU LQ, XIONG T, et al. The role of forkhead box M1-methionine adenosyltransferase 2 A/2B axis in liver inflammation and fibrosis[J]. Nat Commun, 2024, 15( 1): 8388. DOI: 10.1038/s41467-024-52527-8. [55] CHENG FY, SU SY, ZHU XF, et al. Leptin promotes methionine adenosyltransferase 2A expression in hepatic stellate cells by the downregulation of E2F-4 via the β-catenin pathway[J]. FASEB J, 2020, 34( 4): 5578- 5589. DOI: 10.1096/fj.201903021RR. [56] TONG GZ, CHEN XX, LEE J, et al. Fibroblast growth factor 18 attenuates liver fibrosis and HSCs activation via the SMO-LATS1-YAP pathway[J]. Pharmacol Res, 2022, 178: 106139. DOI: 10.1016/j.phrs.2022.106139. [57] YAN GJ, LIN Y, SU HJ, et al. Association between glycolysis and mitochondrial dysfunction and its potential value in liver diseases[J]. J Clin Hepatol, 2022, 38( 8): 1931- 1936. DOI: 10.3969/j.issn.1001-5256.2022.08.042.颜耿杰, 林镛, 苏会吉, 等. 糖酵解与线粒体功能障碍的关系及其在肝脏疾病中的潜在价值[J]. 临床肝胆病杂志, 2022, 38( 8): 1931- 1936. DOI: 10.3969/j.issn.1001-5256.2022.08.042. -



 PDF下载 ( 917 KB)
PDF下载 ( 917 KB)

 下载:
下载:


