改善铜过载所致线粒体功能障碍防治代谢相关脂肪性肝病的研究进展
DOI: 10.12449/JCH251223
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:方禹彤负责文章的构思与设计,论文撰写;陈依凡、郭碧玉、杨惠栾、白雨桐负责论文修订与审校;姚政、杨晓密指导撰写文章并最后定稿。
Research advances in the prevention and treatment of metabolic associated fatty liver disease by alleviating copper overload-induced mitochondrial dysfunction
-
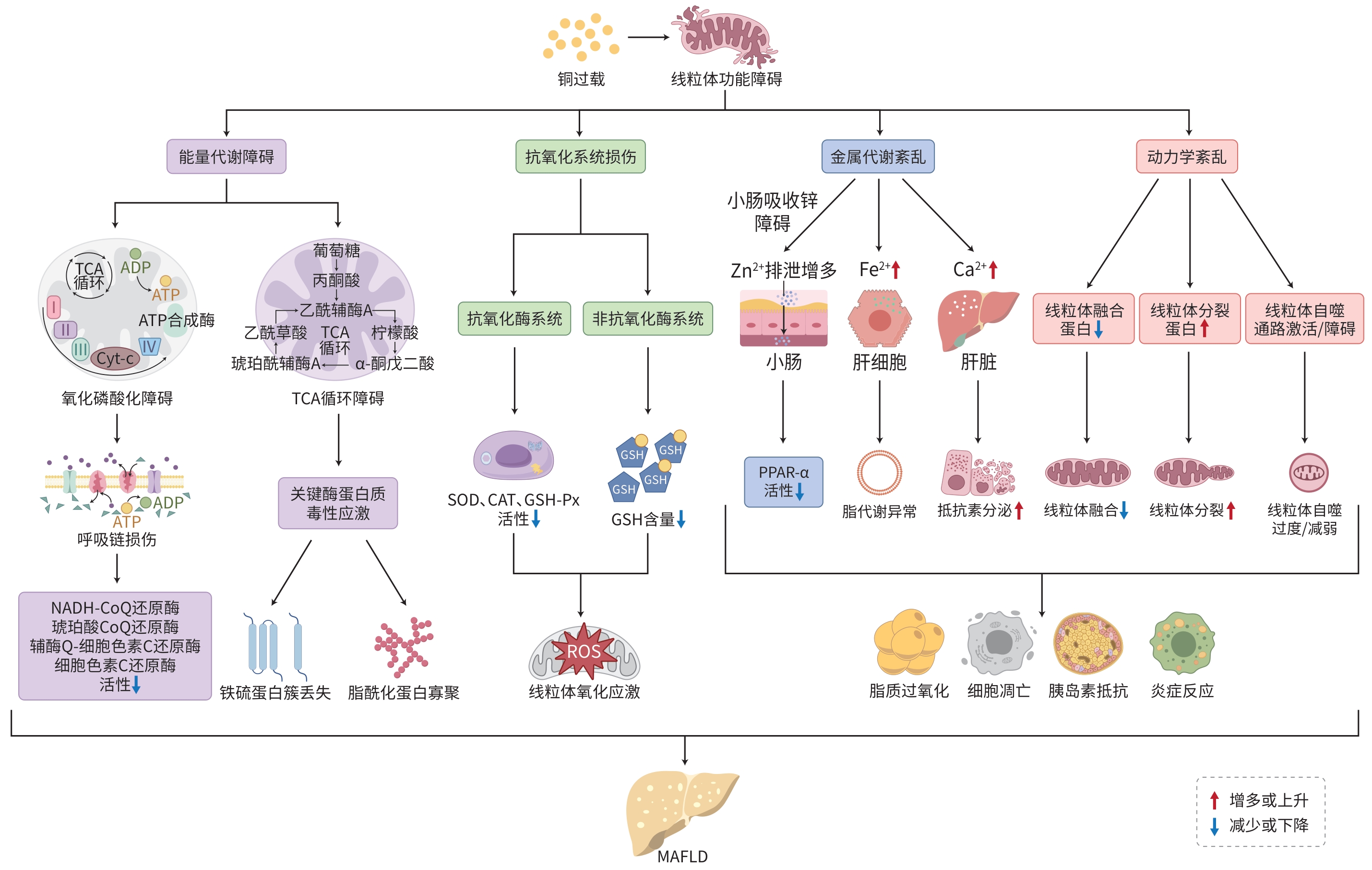
摘要: 铜是人体所必需的微量元素之一,以辅酶或含铜蛋白形式参与线粒体呼吸、能量代谢和抗氧化等过程。细胞内铜蓄积主要表现为铜在线粒体内的异常积聚,导致线粒体形态和功能出现异常。代谢相关脂肪性肝病(MAFLD)的病理进程与线粒体功能障碍密切相关。研究已证实铜过载可以促进MAFLD的发生发展,但关于铜过载导致线粒体功能障碍引发MAFLD的相关机制和药物研究进展,目前尚缺乏系统性总结。基于此,本文系统综述了铜过载引发线粒体功能障碍导致MAFLD的相关机制及药物研究进展,以期为MAFLD进一步的研究和治疗提供理论参考。Abstract: Copper is one of the essential trace elements in the human body and participates in mitochondrial respiration, energy metabolism, and antioxidant processes in the form of coenzymes or copper-containing proteins. Intracellular copper accumulation mainly manifests as abnormal copper accumulation within mitochondria, leading to abnormalities in mitochondrial morphology and function. the pathological process of metabolic associated fatty liver disease (MAFLD) is closely associated with mitochondrial dysfunction. Studies have confirmed that copper overload can promote the development and progression of MAFLD; however, there is still a lack of systematic summarization of the mechanisms by which copper overload induces mitochondrial dysfunction and leads to MAFLD and related advances in drug research. In this context, this article reviews the research advances in the mechanisms by which copper overload induces mitochondrial dysfunction and leads to MAFLD, as well as related advances in drug research, in order to provide a theoretical reference for further investigation and treatment of MAFLD.
-
Key words:
- Metabolic Associated Fatty Liver Disease /
- Mitochondria /
-
Copper
-
[1] ZHOU Y, ZHANG ZW, WANG JQ. Discussion on the prevention and treatment of non-alcoholic fatty liver disease with traditional Chinese medicine from“two-hit” theory[J]. Guid J Tradit Chin Med Pharm, 2017, 23( 18): 109- 111. DOI: 10.13862/j.cnki.cn43-1446/r.2017.18.034.周雨, 张智伟, 王京奇. 从“二次打击”学说探讨中药防治非酒精性脂肪肝的研究进展[J]. 中医药导报, 2017, 23( 18): 109- 111. DOI: 10.13862/j.cnki.cn43-1446/r.2017.18.034. [2] SU JF, JIANG W. Impact of the“two hit theory” on nonalcoholic fatty liver disease[J]. Acta Med Sin, 2015, 28( 2): 141- 144.苏剑锋, 江伟.“二次打击”对非酒精性脂肪肝的影响[J]. 华夏医学, 2015, 28( 2): 141- 144. [3] BUZZETTI E, PINZANI M, TSOCHATZIS EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease(NAFLD)[J]. Metabolism, 2016, 65( 8): 1038- 1048. DOI: 10.1016/j.metabol.2015.12.012. [4] CHEN LY, MIN JX, WANG FD. Copper homeostasis and cuproptosis in health and disease[J]. Signal Transduct Target Ther, 2022, 7: 378. DOI: 10.1038/s41392-022-01229-y. [5] LEI HT, WANG HD, WANG JH, et al. Progress in regulation mechanism of copper death[J]. Chin J Pathophysiol, 2023, 39( 8): 1491- 1498. DOI: 10.3969/j.issn.1000-4718.2023.08.018.雷海桃, 王海东, 王金海, 等. 铜死亡调控机制的研究进展[J]. 中国病理生理杂志, 2023, 39( 8): 1491- 1498. DOI: 10.3969/j.issn.1000-4718.2023.08.018. [6] NAM E, HAN J, SUH JM, et al. Link of impaired metal ion homeostasis to mitochondrial dysfunction in neurons[J]. Curr Opin Chem Biol, 2018, 43: 8- 14. DOI: 10.1016/j.cbpa.2017.09.009. [7] ZHU ZW, YAO L. Research progress in investigating the molecular mechanism of copper homeostasis[J]. Chin Bull Life Sci, 2012, 24( 8): 847- 857. DOI: 10.13376/j.cbls/2012.08.022.朱志兀, 姚琳. 铜离子稳态平衡分子机理研究进展[J]. 生命科学, 2012, 24( 8): 847- 857. DOI: 10.13376/j.cbls/2012.08.022. [8] TSVETKOV P, COY S, PETROVA B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins[J]. Science, 2022, 375( 6586): 1254- 1261. DOI: 10.1126/science.abf0529. [9] TAN WJ, ZHANG JL, CHEN L, et al. Copper homeostasis and cuproptosis-related genes: Therapeutic perspectives in non-alcoholic fatty liver disease[J]. Diabetes Obes Metab, 2024, 26( 11): 4830- 4845. DOI: 10.1111/dom.15846. [10] ASCHNER M, SKALNY AV, LU RZ, et al. Mitochondrial pathways of copper neurotoxicity: Focus on mitochondrial dynamics and mitophagy[J]. Front Mol Neurosci, 2024, 17: 1504802. DOI: 10.3389/fnmol.2024.1504802. [11] LI LR, YI Y, SHU XW, et al. The correlation between serum copper and non-alcoholic fatty liver disease in American adults: An analysis based on NHANES 2011 to 2016[J]. Biol Trace Elem Res, 2024, 202( 10): 4398- 4409. DOI: 10.1007/s12011-023-04029-9. [12] ZHANG CY, YANG M. Current options and future directions for NAFLD and NASH treatment[J]. Int J Mol Sci, 2021, 22( 14): 7571. DOI: 10.3390/ijms22147571. [13] QU SY, LIU SZ, ZHANG ZQ, et al. Copper in the diet affects lipid metabolism in mice[J]. Basic Clin Med, 2019, 39( 6): 776- 780. DOI: 10.16352/j.issn.1001-6325.2019.06.003.瞿思遥, 刘思哲, 张祝琴, 等. 饮食中的铜影响小鼠脂质代谢[J]. 基础医学与临床, 2019, 39( 6): 776- 780. DOI: 10.16352/j.issn.1001-6325.2019.06.003. [14] PAN YX. Effects and mechanisms of copper and cadmium exposure on lipid metabolism in zebrafish Danio rerio[D]. Wuhan: Huazhong Agricultural University, 2018. DOI: 10.27158/d.cnki.ghznu.2018.000248.潘亚雄. 水体铜和镉暴露对斑马鱼脂肪代谢的影响及机理研究[D]. 武汉: 华中农业大学, 2018. DOI: 10.27158/d.cnki.ghznu.2018.000248. [15] WU CT, LIU XX, ZHONG LX, et al. Identification of cuproptosis-related genes in nonalcoholic fatty liver disease[J]. Oxid Med Cell Longev, 2023, 2023: 9245667. DOI: 10.1155/2023/9245667. [16] XU YC, XU YH, ZHAO T, et al. Waterborne Cu exposure increased lipid deposition and lipogenesis by affecting Wnt/β-catenin pathway and the β-catenin acetylation levels of grass carp Ctenopharyngodon idella[J]. Environ Pollut, 2020, 263( Pt B): 114420. DOI: 10.1016/j.envpol.2020.114420. [17] ZHU SY, ZHOU WQ, NIU YY, et al. COX17 restricts renal fibrosis development by maintaining mitochondrial copper homeostasis and restoring complex IV activity[J]. Acta Pharmacol Sin, 2023, 44( 10): 2091- 2102. DOI: 10.1038/s41401-023-01098-3. [18] ZHU MQ, XIE X, LIAO QC, et al. Mechanism of cuproptosis and its role in liver diseases[J]. J Clin Hepatol, 2024, 40( 11): 2332- 2337. DOI: 10.12449/JCH241131.朱明强, 谢星, 廖启成, 等. 铜死亡的发生机制及在肝脏疾病中的作用[J]. 临床肝胆病杂志, 2024, 40( 11): 2332- 2337. DOI: 10.12449/JCH241131. [19] LIU T, LIU YL, ZHANG FY, et al. Copper homeostasis dysregulation promoting cell damage and the association with liver diseases[J]. Chin Med J, 2023, 136( 14): 1653- 1662. DOI: 10.1097/CM9.00000000000-02697. [20] LI C, YANG W, DENG YF, et al. Research Progresson copper metabolism disorders-mediated non-Alcoholic Fatty liver disease combinedwith srcoa Penia[J]. Prog Physiol Sci, 2024, 55( 2): 163- 170. DOI: 10.20059/j.cnki.pps.2023.10.1090.李畅, 杨威, 邓云锋, 等. 铜代谢紊乱介导非酒精性脂肪性肝病合并肌少症的研究进展[J]. 生理科学进展, 2024, 55( 2): 163- 170. DOI: 10.20059/j.cnki.pps.2023.10.1090. [21] JOMOVA K, VALKO M. Advances in metal-induced oxidative stress and human disease[J]. Toxicology, 2011, 283( 2-3): 65- 87. DOI: 10.1016/j.tox.2011.03.001. [22] SCHEIBER I, DRINGEN R, MERCER JFB. Copper: Effects of deficiency and overload[J] Met Ions Life Sci, 2013, 13: 359- 387. DOI: 10.1007/978-94-007-7500-8_11. [23] CAO H, SU R, HU G, et al. In vivo effects of high dietary copper levels on hepatocellular mitochondrial respiration and electron transport chain enzymes in broilers[J]. Br Poult Sci, 2016, 57( 1): 63- 70. DOI: 10.1080/00071668.2015.1127895. [24] GUTIÉRREZ-GARCÍA R, DEL POZO T, SUAZO M, et al. Physiological copper exposure in Jurkat cells induces changes in the expression of genes encoding cholesterol biosynthesis proteins[J]. BioMetals, 2013, 26( 6): 1033- 1040. DOI: 10.1007/s10534-013-9680-9. [25] LIU Y, YANG HR, SONG Z, et al. Copper excess in liver HepG2 cells interferes with apoptosis and lipid metabolic signaling at the protein level[J]. Turk J Gastroenterol, 2014, 25( Suppl 1): 116- 121. DOI: 10.5152/tjg.2014.5064. [26] OZCELIK D, OZARAS R, GUREL Z, et al. Copper-mediated oxidative stress in rat liver[J]. Biol Trace Elem Res, 2003, 96( 1-3): 209- 215. DOI: 10.1385/BTER: 96: 1-3: 209. [27] ZHONG GL, LI YX, MA FY, et al. Copper exposure induced chicken hepatotoxicity: Involvement of ferroptosis mediated by lipid peroxidation, ferritinophagy, and inhibition of FSP1-CoQ10 and Nrf2/SLC7A11/GPX4 axis[J]. Biol Trace Elem Res, 2024, 202( 4): 1711- 1721. DOI: 10.1007/s12011-023-03773-2. [28] YU WL, LIAO JZ, YANG F, et al. Chronic tribasic copper chloride exposure induces rat liver damage by disrupting the mitophagy and apoptosis pathways[J]. Ecotoxicol Environ Saf, 2021, 212: 111968. DOI: 10.1016/j.ecoenv.2021.111968. [29] WU LL, GONG W, WU G, et al. Correlation of plasma trace elements and non-alcoholic fatty liver disease[J]. Jiangsu Med J, 2017, 43( 5): 311- 314. DOI: 10.19460/j.cnki.0253-3685.2017.05.003.吴林林, 龚伟, 吴钢, 等. 血浆微量元素与非酒精性脂肪性肝病的相关性[J]. 江苏医药, 2017, 43( 5): 311- 314. DOI: 10.19460/j.cnki.0253-3685.2017.05.003. [30] DOGUER C, HA JH, COLLINS JF. Intersection of iron and copper metabolism in the mammalian intestine and liver[J]. Compr Physiol, 2018, 8( 4): 1433- 1461. DOI: 10.1002/cphy.c170045. [31] ZHAO JY, LI YW, LI L. The role of iron and hepcidin in hepatic fibrosis[J]. Prog Physiol Sci, 2010, 41( 3): 183- 188.赵晋英, 李艳伟, 李琳. 铁和铁调素在肝纤维化中的作用[J]. 生理科学进展, 2010, 41( 3): 183- 188. [32] OESTREICHER P, COUSINS RJ. Copper and zinc absorption in the rat: Mechanism of mutual antagonism[J]. J Nutr, 1985, 115( 2): 159- 166. DOI: 10.1093/jn/115.2.159. [33] HIMOTO T, MASAKI T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease[J]. Nutrients, 2018, 10( 1): 88. DOI: 10.3390/nu10010088. [34] GUO XH, WANG JH, DUAN XL, et al. Metal ion metabolism: New ideas for the traditional Chinese medicine prevention and treatment of chronic liver disease[J]. J Clin Hepatol, 2024, 40( 7): 1498- 1504. DOI: 10.12449/JCH240732.郭新华, 王佳慧, 段雪琳, 等. 金属离子代谢: 慢性肝病中医药防治新思路[J]. 临床肝胆病杂志, 2024, 40( 7): 1498- 1504. DOI: 10.12449/JCH240732. [35] WANG X, AN P, GU ZL, et al. Mitochondrial metal ion transport in cell metabolism and disease[J]. Int J Mol Sci, 2021, 22( 14): 7525. DOI: 10.3390/ijms22147525. [36] ZHANG C, MIAO JR, FAN X. The role of circadian clock-controlled mitochondrial dynamics in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2024, 40( 8): 1670- 1676. DOI: 10.12449/JCH240826.张策, 苗嘉芮, 樊旭. 生物钟调控的线粒体动力学在非酒精性脂肪性肝病中的作用[J]. 临床肝胆病杂志, 2024, 40( 8): 1670- 1676. DOI: 10.12449/JCH240826. [37] VAN TOL AMARAL GUERRA SM, CORDEIRO KOPPE DE FRANÇA L, NETO DA SILVA K, et al. Copper dyshomeostasis and its relationship to AMPK activation, mitochondrial dynamics, and biogenesis of mitochondria: A systematic review of in vivo studies[J]. J Trace Elem Med Biol, 2024, 86: 127549. DOI: 10.1016/j.jtemb.2024.127549. [38] WANG YH, ZHU YQ, CUI HM, et al. Effects of CuSO4 on hepatic mitochondrial function, biogenesis and dynamics in mice[J]. Environ Toxicol, 2024, 39( 4): 2208- 2217. DOI: 10.1002/tox.24085. [39] YANG F, LIAO JZ, YU WL, et al. Exposure to copper induces mitochondria-mediated apoptosis by inhibiting mitophagy and the PINK1/parkin pathway in chicken(Gallus gallus) livers[J]. J Hazard Mater, 2021, 408: 124888. DOI: 10.1016/j.jhazmat.2020.124888. [40] HRUBY M, MARTÍNEZ IIS, STEPHAN H, et al. Chelators for treatment of iron and copper overload: Shift from low-molecular-weight compounds to polymers[J]. Polymers, 2021, 13( 22): 3969. DOI: 10.3390/polym1-3223969. [41] ESPINOZA A, LE BLANC S, OLIVARES M, et al. Iron, copper, and zinc transport: Inhibition of divalent metal transporter 1(DMT1) and human copper transporter 1(hCTR1) by shRNA[J]. Biol Trace Elem Res, 2012, 146( 2): 281- 286. DOI: 10.1007/s12011-011-9243-2. [42] FATHI M, ALAVINEJAD P, HAIDARI Z, et al. The effects of zinc supplementation on metabolic profile and oxidative stress in overweight/obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial[J]. J Trace Elem Med Biol, 2020, 62: 126635. DOI: 10.1016/j.jtemb.2020.126635. [43] BALDARI S, DI ROCCO G, TOIETTA G. Current biomedical use of copper chelation therapy[J]. Int J Mol Sci, 2020, 21( 3): 1069. DOI: 10.3390/ijms21031069. [44] CAPRIOTTI G, VARANI M, LAURI C, et al. Copper-64 labeled nanoparticles for positron emission tomography imaging: A review of the recent literature[J]. Q J Nucl Med Mol Imaging, 2020, 64( 4): 346- 355. DOI: 10.23736/S1824-4785.20.03315-4. [45] ZHOU XX, LIAO J, LIU YJ, et al. Symptom aggravation after withdrawal of metal chelating agent therapy in patients with Wilson’s disease[J]. Brain Behav, 2023, 13( 9): e3170. DOI: 10.1002/brb3.3170. [46] MANLEY OM, ROSENZWEIG AC. Copper-chelating natural products[J]. J Biol Inorg Chem, 2025, 30( 2): 111- 124. DOI: 10.1007/s00775-025-02099-9. [47] SANTINI SJ, TARANTINO G, IEZZI A, et al. Copper-catalyzed dicarbonyl stress in NAFLD mice: Protective effects of Oleuropein treatment on liver damage[J]. Nutr Metab, 2022, 19( 1): 9. DOI: 10.1186/s12986-022-00641-z. [48] ANTONUCCI L, PORCU C, IANNUCCI G, et al. Non-alcoholic fatty liver disease and nutritional implications: Special focus on copper[J]. Nutrients, 2017, 9( 10): 1137. DOI: 10.3390/nu9101137. [49] WAN XH, LI YW, LUO XP, et al. Curcumin attenuated the lipid peroxidation and apoptotic liver injury in copper-overloaded rats[J]. Chin J Pediatr, 2007, 45( 8): 604- 608. DOI: 10.3760/j.issn: 0578-1310.2007.08.011.万小华, 李毓雯, 罗小平, 等. 铜负荷大鼠肝脏脂质过氧化和凋亡损伤及姜黄素的保护作用[J]. 中华儿科杂志, 2007, 45( 8): 604- 608. DOI: 10.3760/j.issn: 0578-1310.2007.08.011. [50] YANG CL, WU J, CHEN YK. Protective effect of Salvia miltiorrhiza on high-copper diet-induced liver injury in rats[J]. J Tradit Chin Vet Med, 2016, 35( 6): 13- 16. DOI: 10.13823/j.cnki.jtcvm.2016.06.003.杨成林, 邬静, 陈宇科. 丹参对铜诱导大鼠肝损伤的保护作用研究[J]. 中兽医医药杂志, 2016, 35( 6): 13- 16. DOI: 10.13823/j.cnki.jtcvm.2016.06.003. -



 PDF下载 ( 1107 KB)
PDF下载 ( 1107 KB)

 下载:
下载:


