基于网络药理学探讨柴胡皂苷e联合吉非替尼治疗胆管癌的作用机制
DOI: 10.12449/JCH251221
Mechanism of action of saikosaponin e combined with gefitinib in treatment of cholangiocarcinoma: A study based on network pharmacology
-
摘要:
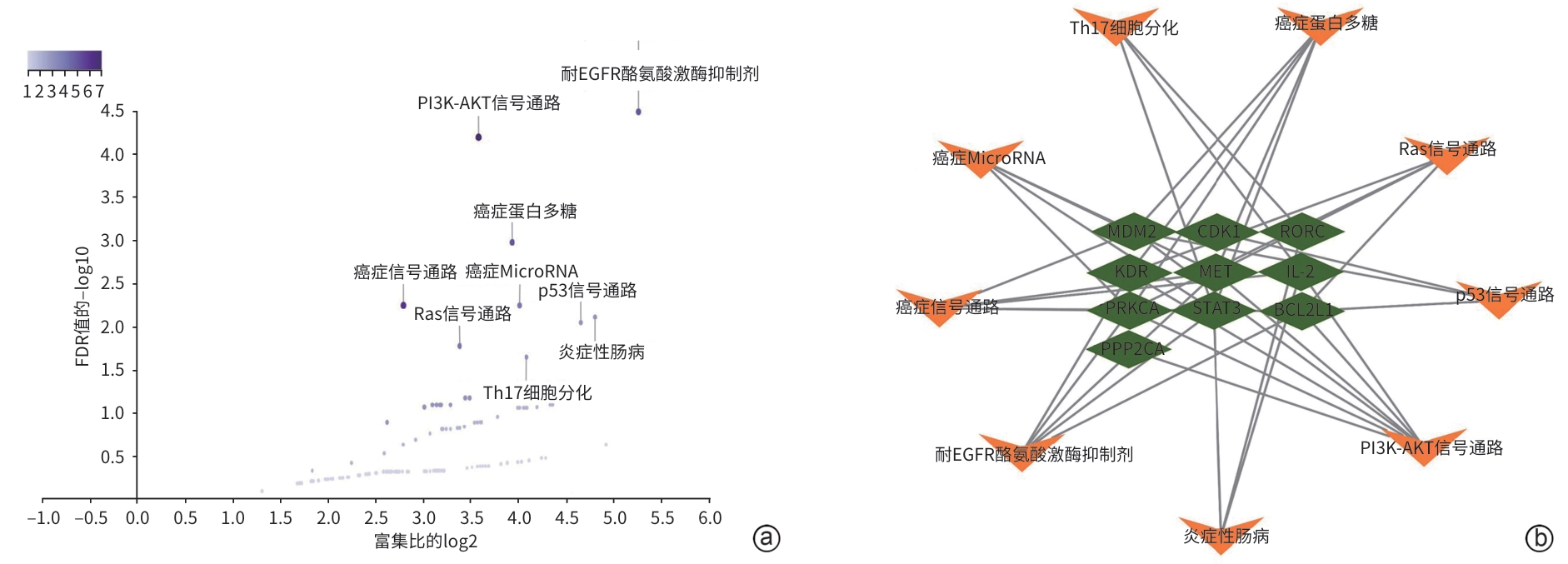
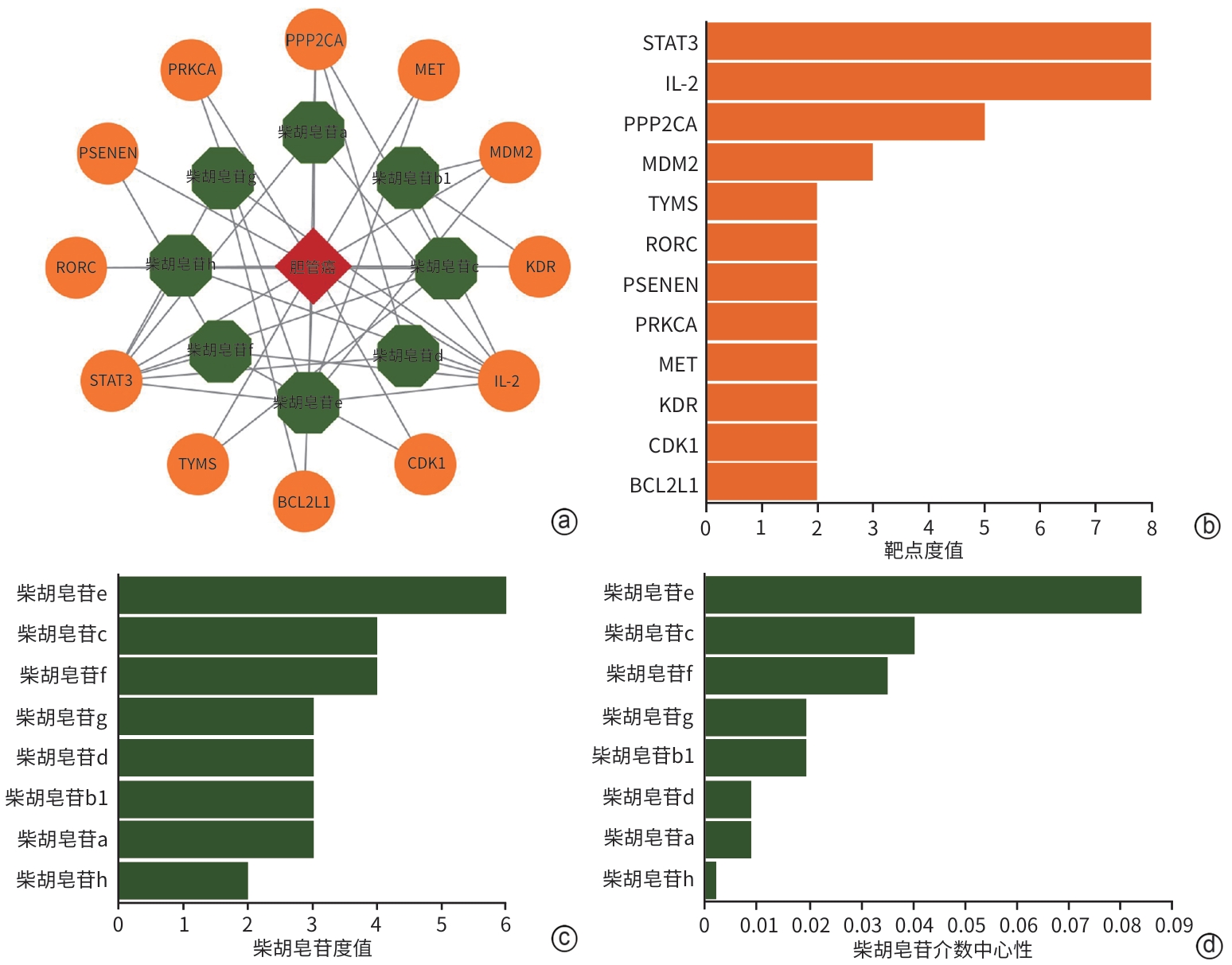
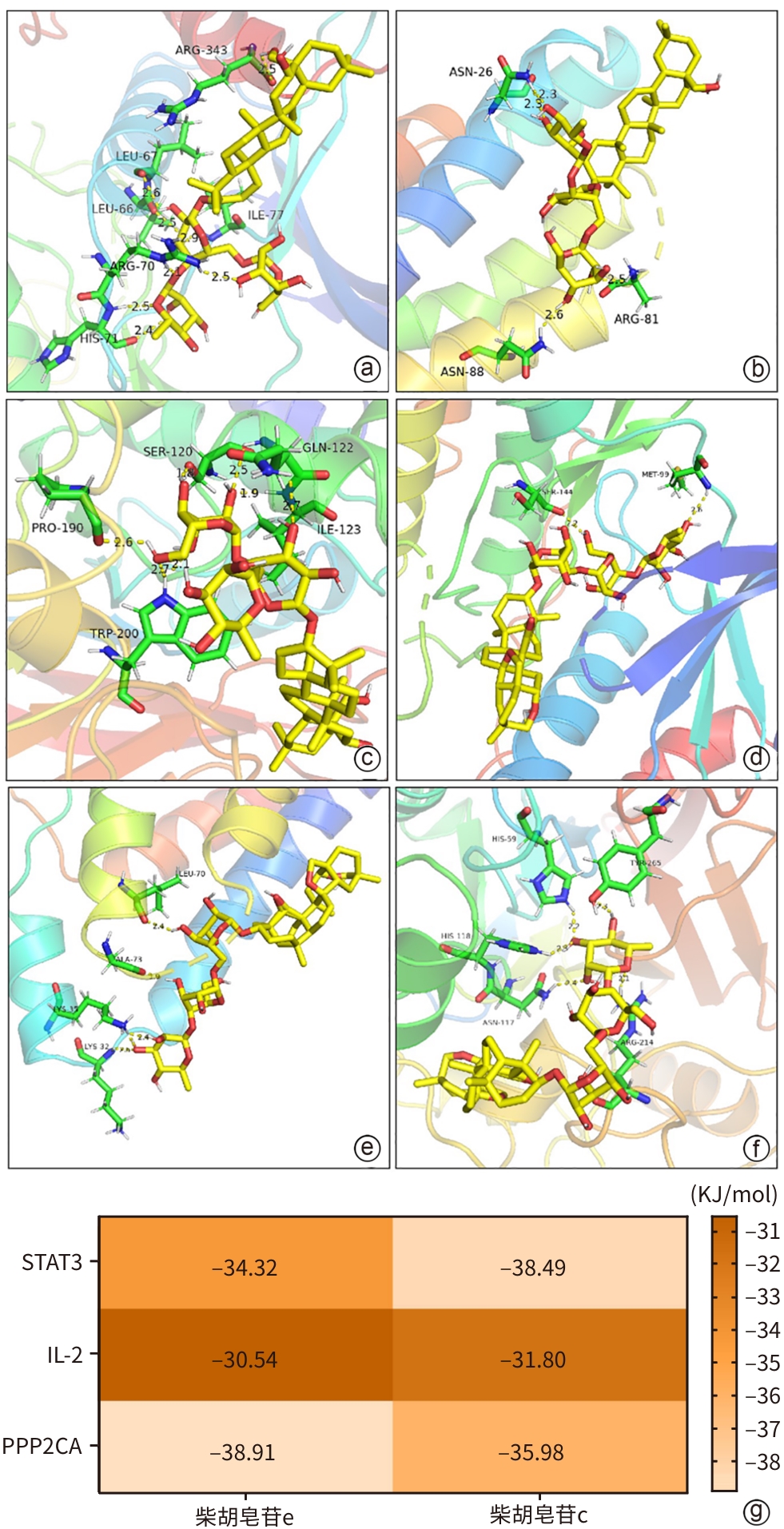
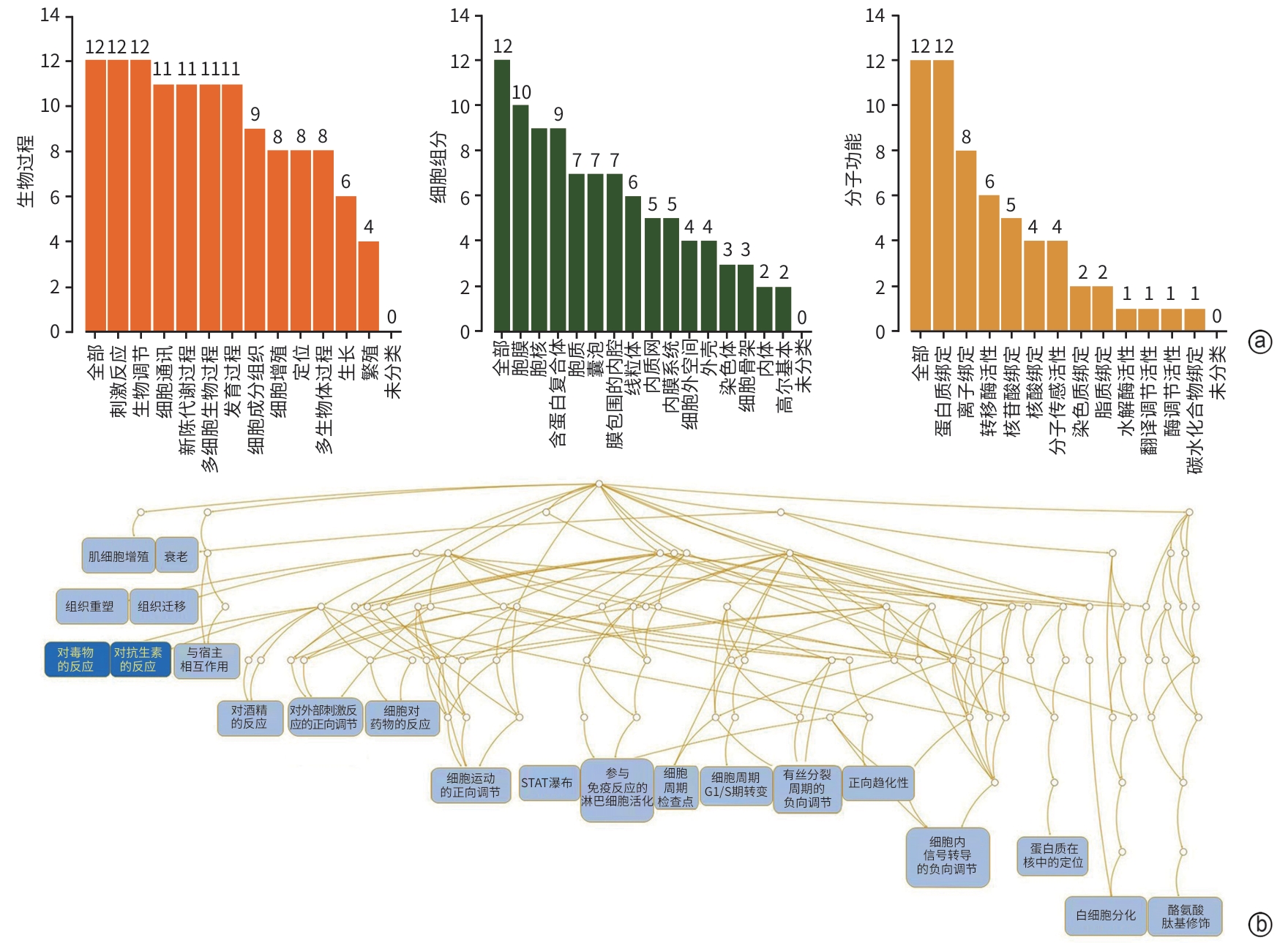
目的 通过网络药理学和细胞实验探讨柴胡皂苷e联合吉非替尼抗胆管癌的药理作用和分子机制。 方法 通过SwissTargetPrediction数据库获得柴胡皂苷a、b1、c、d、e、f、g、h的药物作用靶点;通过GeneCards数据库获得胆管癌的靶点;取交集靶点导入STRING数据库构建蛋白质-蛋白质互作网络;利用WEB-based GEne SeT AnaLysis Toolkit数据库进行GO和KEGG通路富集分析并构建靶点-通路网络,利用Pathview R包标注关键通路涵盖的靶点;利用CytoScape 3.7.2构建药物-疾病-靶点网络并筛选药效较强的化合物与关键靶点分子对接;人胆管癌RBE细胞随机分为对照组、柴胡皂苷e组、吉非替尼组、柴胡皂苷e和吉非替尼联用组,MTT、EdU、划痕实验、荧光探针法及微量法检测细胞增殖、迁移及活性氧(ROS)、丙二醛(MDA)生成;Western Blotting检测PI3K、p-PI3K、AKT、p-AKT蛋白表达。半数抑制浓度(IC50)采用Logistic回归计算,计量资料多组间比较采用单因素方差分析和重复测量资料方差分析,进一步两两比较采用Tukey检验,单独效应比较采用LSD-t检验。 结果 获得柴胡皂苷靶点34个,胆管癌靶点1 815个,交集靶点12个,拓扑分析后显示,柴胡皂苷e和c抗胆管癌作用较强,STAT3、IL-2和PPP2CA为关键靶点,柴胡皂苷e、c分别与STAT3、IL-2、PPP2CA相应氨基酸位点形成氢键对接。GO富集分析得到生物过程条目13个,细胞组分条目16个,分子功能条目13个;KEGG通路分析得到9条通路,PI3K-AKT和耐EGFR酪氨酸激酶抑制剂为关键信号通路,涵盖了STAT3、IL-2和PPP2CA等多个靶点;柴胡皂苷e 和吉非替尼作用于人胆管癌RBE细胞24 h的IC50分别为16.89 μmol/L 和27.49 μmol/L;与对照组相比,柴胡皂苷e作用24 h可显著降低人胆管癌RBE细胞的增殖率(53.46%±6.42% vs 100.00%±6.00%,P<0.000 1)和迁移率(12.06%±1.76% vs 16.01%±1.89%,P<0.05),诱导ROS和MDA产生增多(P值均<0.05),下调p-AKT蛋白表达(P<0.05),且与吉非替尼联用效果更显著(P值均<0.05)。 结论 本研究显示柴胡皂苷e和吉非替尼通过抑制PI3K-AKT信号通路发挥抗胆管癌作用,为柴胡皂苷类药物的深入研究和临床应用提供一定的理论支持和科学依据。 Abstract:Objective To investigate the pharmacological effect and molecular mechanism of saikosaponin e combined with gefitinib in the treatment of cholangiocarcinoma based on network pharmacology and cell experiment. Methods SwissTargetPrediction was used to obtain the drug action targets of saikosaponin a, b1, c, d, e, f, g, and h, and GeneCards was used to obtain the targets of cholangiocarcinoma. The intersecting targets of these two groups of targets were imported into STRING to construct a protein-protein interaction network. WEB-based GEne SeT AnaLysis Toolkit was used to perform gene ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and construct a target-pathway network, and Pathview R package was used to label the targets covered by the key pathways. CytoScape 3.7.2 was used to construct a drug-disease-target network, and molecular docking was performed between effective compounds and key targets. Human cholangiocarcinoma RBE cells were randomly divided into control group, saikosaponin e group, gefitinib group, and saikosaponin e+gefitinib group. MTT assay, EdU, scratch assay, and the fluorescence probe method were used to measure the proliferation and migration of RBE cells and the production of reactive oxygen species (ROS) and malondialdehyde (MDA), and Western blotting was used to measure the protein expression levels of phosphatidylinositol 3-kinase (PI3K), phosphorylated PI3K (p-PI3K), protein kinase B (AKT), and phosphorylated AKT (p-AKT). Logistic regression was used to calculate half-maximal inhibitory concentration (IC50); A one-way analysis of variance and repeated measures analysis of variance were used for comparison of continuous data between multiple groups, and the Tukey test was used for further comparison between two groups; the least significant difference t-test was used for comparison of simple effect. Results A total of 34 saikosaponin targets and 1 815 cholangiocarcinoma targets were obtained, resulting in 12 intersecting targets. The topological analysis showed that saikosaponins e and c had a stronger efficacy against cholangiocarcinoma, with the key targets of STAT3, IL-2, and PPP2CA, and saikosaponin e and c could respectively dock with the corresponding amino acid sites of STAT3, IL-2, and PPP2CA by forming hydrogen bonds. The GO functional enrichment analysis obtained 13 biological processes, 16 cellular components, and 13 molecular functions, and the KEGG pathway enrichment analysis obtained 9 pathways, among which PI3K-AKT and EGFR tyrosine kinase inhibitor resistance were the key signaling pathways, covering multiple targets including STAT3, IL-2, and PPP2CA. Saikosaponin e and gefitinib had an IC50 of 16.89 μmol/L and 27.49 μmol/L, respectively, on human cholangiocarcinoma RBE cells at 24 hours of treatment, and compared with the control group, saikosaponin e treatment for 24 hours significantly reduced the proliferation rate (53.46%±6.42% vs 100.00%±6.00%, P<0.000 1) and migration rate (12.06%±1.76% vs 16.01%±1.89%, P<0.05) of human cholangiocarcinoma RBE cells, increased the production of ROS and MDA (both P<0.05), and downregulated the protein expression of p-AKT (P<0.05), while its combination with gefitinib had a significantly greater effect (all P<0.05). Conclusion This study shows that saikosaponin e and gefitinib exert a therapeutic effect on cholangiocarcinoma by inhibiting the PI3K-AKT signaling pathway, which provides theoretical support and a scientific basis for further research and clinical application of saikosaponins. -
Key words:
- Saikosaponins /
- Gefitinib /
- Bile Duct Neoplasms /
- Pharmacology
-
注: a、b,柴胡皂苷e、吉非替尼分别作用24 h的IC50;c、d,柴胡皂苷e和吉非替尼对胆管癌RBE细胞增殖的影响,图c中F=108.30,P<0.000 1,图d中F=72.71,P<0.000 1(胞质为EdU染色,胞核为Hoechst染色,×10);e,柴胡皂苷e和吉非替尼对胆管癌RBE细胞迁移的影响(×10),时间&组别:F=42.96,P<0.000 1,时间:F=362.93,P<0.000 1,组别:F=109.60,P<0.000 1。与对照组比较,*P<0.05,***P<0.001,****P<0.000 1。
图 5 柴胡皂苷e和吉非替尼对胆管癌RBE细胞增殖迁移的影响
Figure 5. Effect of saikosaponin e and gefitinib on the proliferation and migration of RBE cells
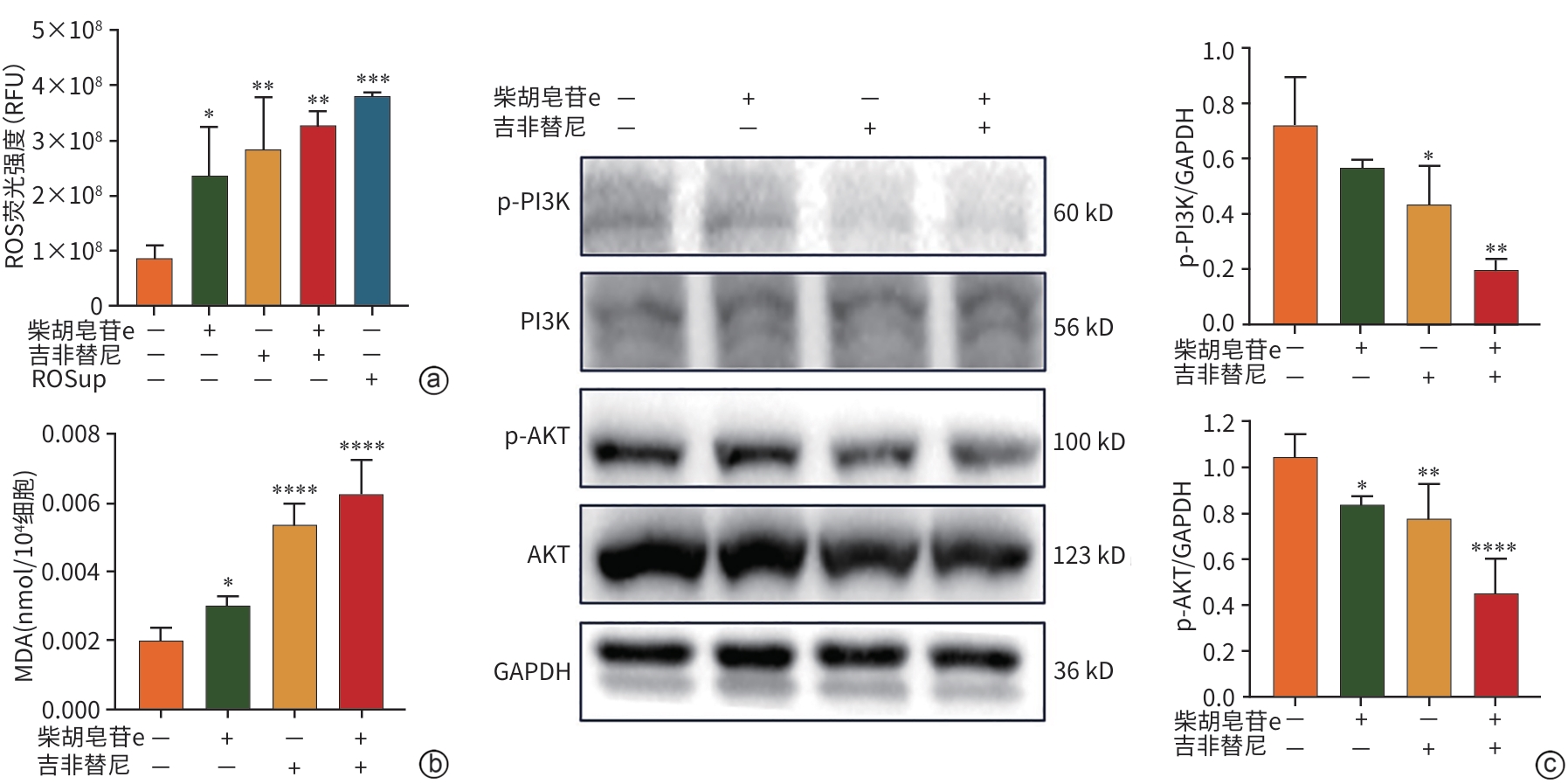
注: a、b,柴胡皂苷e和吉非替尼对ROS、MDA生成的影响,图a中 F=10.36,P=0.001 4,ROSup:ROS诱导剂,RFU:相对荧光单位;图b中F=57.43,P<0.000 1; c,柴胡皂苷e和吉非替尼对p-PI3K和p-AKT表达的影响,p-PI3K分析F=11.56,P=0.002 8,p-AKT分析F=21.2,P<0.000 1。与对照组比较,*P<0.05,**P<0.01,****P<0.000 1。
图 6 柴胡皂苷e和吉非替尼对ROS和MDA生成及p-PI3K和p-AKT表达的影响
Figure 6. Effect of saikosaponin e and gefitinib on the production of ROS and MDA as well as the expression of p-PI3K and p-AKT
表 1 柴胡皂苷e对胆管癌RBE细胞增殖率的影响
Table 1. Effect of saikosaponin e on the proliferation rate of RBE cells
浓度 12 h细胞
增殖率(%)24 h细胞
增殖率(%)48 h细胞
增殖率(%)0 μmol/L 100.00±2.71 100.00±1.74 100.00±2.30 2 μmol/L 93.21±1.411) 84.19±4.691) 77.78±2.571) 4 μmol/L 81.70±1.321) 74.70±4.371) 68.81±2.421) 8 μmol/L 76.51±2.071) 67.30±3.921) 61.26±2.681) 16 μmol/L 73.69±4.141) 52.96±4.111) 43.39±2.161) 32 μmol/L 65.00±1.171) 35.29±3.321) 13.44±1.781) 注:时间&浓度:F=98.50,P<0.000 1;时间:F=807.33,P<0.000 1;浓度:F=1 176.30,P<0.000 1。与同一时间的0 μmol/L比较,1)P<0.000 1。
表 2 吉非替尼对胆管癌RBE细胞增殖率的影响
Table 2. Effect of gefitinib on the proliferation rate of RBE cells
浓度 12 h细胞
增殖率(%)24 h细胞
增殖率(%)48 h细胞
增殖率(%)0 μmol/L 100.00±5.83 100.00±3.88 100.00±3.30 12.5 μmol/L 64.59±4.041) 51.50±3.941) 36.15±2.501) 25 μmol/L 60.18±2.561) 47.02±5.221) 31.30±2.271) 50 μmol/L 57.14±3.191) 38.26±4.891) 29.50±1.041) 100 μmol/L 53.59±8.281) 36.59±1.911) 23.59±2.601) 200 μmol/L 47.34±2.971) 33.98±2.891) 19.45±5.301) 注:时间&浓度:F=16.87,P<0.000 1;时间:F=396.66,P<0.000 1;浓度:F=1 423.68,P<0.000 1。与同一时间的0 μmol/L比较,1)P<0.000 1。
-
[1] MORIS D, PALTA M, KIM C, et al. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians[J]. CA A Cancer J Clin, 2023, 73( 2): 198- 222. DOI: 10.3322/caac.21759. [2] QURASHI M, VITHAYATHIL M, KHAN SA. Epidemiology of cholangiocarcinoma[J]. Eur J Surg Oncol, 2025, 51( 2): 107064. DOI: 10.1016/j.ejso.2023.107064. [3] BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. [4] SARCOGNATO S, SACCHI D, FASSAN M, et al. Cholangiocarcinoma[J]. Pathologica, 2021, 113( 3): 158- 169. DOI: 10.32074/1591-951x-252. [5] CHEN ZM, CHEN JH. Advances in precision diagnosis and treatment of cholangiocarcinoma[J]. Chin J Clin Pharmacol Ther, 2025, 30( 2): 159- 170. DOI: 10.12092/j.issn.1009-2501.2025.02.002.陈祯美, 陈进宏. 胆管癌精准诊疗进展及前沿[J]. 中国临床药理学与治疗学, 2025, 30( 2): 159- 170. DOI: 10.12092/j.issn.1009-2501.2025.02.002. [6] ELVEVI A, LAFFUSA A, SCARAVAGLIO M, et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review[J]. Ann Hepatol, 2022, 27( 5): 100737. DOI: 10.1016/j.aohep.2022.100737. [7] LI YH, YU JF, ZHANG YJ, et al. Advances in targeted therapy of cholangiocarcinoma[J]. Ann Med, 2024, 56( 1): 2310196. DOI: 10.1080/07853890.2024.2310196. [8] LIU Y, LIU LX. New advances in diagnosis and treatment of advanced cholangiocarcinoma[J]. Chin J Dig Surg, 2025, 24( 7): 855- 861. DOI: 10.3760/cma.j.cn115610-20250606-00261.刘尧, 刘连新. 晚期胆管癌诊断与治疗新进展[J]. 中华消化外科杂志, 2025, 24( 7): 855- 861. DOI: 10.3760/cma.j.cn115610-20250606-00261. [9] WANG HQ, ZHOU QY, LI BQ, et al. Chemical compositions and pharmacological effects of Chinese thorowax root: Review[J]. Jilin J Chin Med, 2024, 44( 1): 96- 100. DOI: 10.13463/j.cnki.jlzyy.2024.01.022.王海强, 周千瑶, 李冰琪, 等. 柴胡化学成分及药理作用研究进展[J]. 吉林中医药, 2024, 44( 1): 96- 100. DOI: 10.13463/j.cnki.jlzyy.2024.01.022. [10] LI X, LI XY, HUANG NN, et al. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins[J]. Phytomedicine, 2018, 50: 73- 87. DOI: 10.1016/j.phymed.2018.09.174. [11] QUE RY, LI Y. Advances in mechanisms of saikosaponins preventing and treating hepatocellular carcinoma[J]. J Liaoning Univ Tradit Chin Med, 2014, 16( 2): 128- 131. DOI: 10.13194/j.issn.1673-842x.2014.02.044.阙任烨, 李勇. 柴胡皂苷防治原发性肝癌机制研究进展[J]. 辽宁中医药大学学报, 2014, 16( 2): 128- 131. DOI: 10.13194/j.issn.1673-842x.2014.02.044. [12] ZHANG P, ZHANG DF, ZHOU WA, et al. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine[J]. Brief Bioinform, 2023, 25( 1): bbad518. DOI: 10.1093/bib/bbad518. [13] ZHAO FY, LI S, WANG XQ, et al. Molecular mechanism of traditional Chinese medicine active components in regulating glucose metabolism against cholangiocarcinoma[J]. J Clin Hepatol, 2024, 40( 8): 1704- 1708. DOI: 10.12449/JCH240832.赵方言, 李姗, 王祥麒, 等. 中药活性成分调控糖代谢抗胆管癌的分子机制[J]. 临床肝胆病杂志, 2024, 40( 8): 1704- 1708. DOI: 10.12449/JCH240832. [14] BAI JZ, JIA LQ, CHEN DM, et al. Research progress on medication patterns and pharmacology of traditional Chinese medicine for treating cholangiocarcinoma based on syndrome differentiation[J]. Chin Tradit Herb Drugs, 2023, 54( 24): 8228- 8240. DOI: 10.7501/j.issn.0253-2670.2023.24.028.白金钊, 贾立群, 陈冬梅, 等. 中医辨证施治胆管癌用药规律及药理学研究进展[J]. 中草药, 2023, 54( 24): 8228- 8240. DOI: 10.7501/j.issn.0253-2670.2023.24.028. [15] ZHU YC, LAI Y. Pharmacological properties and derivatives of saikosaponins: A review of recent studies[J]. J Pharm Pharmacol, 2023, 75( 7): 898- 909. DOI: 10.1093/jpp/rgad052. [16] XIAO X, GAO C. Saikosaponins targeting programmed cell death as anticancer agents: Mechanisms and future perspectives[J]. Drug Des Devel Ther, 2024, 18: 3697- 3714. DOI: 10.2147/DDDT.S470455. [17] ZHAO X, LIU JY, GE SS, et al. Saikosaponin a inhibits breast cancer by regulating Th1/Th2 balance[J]. Front Pharmacol, 2019, 10: 624. DOI: 10.3389/fphar.2019.00624. [18] YOU M, FU JM, LV XZ, et al. Saikosaponin b2 inhibits tumor angiogenesis in liver cancer via down-regulation of VEGF/ERK/HIF-1α signaling[J]. Oncol Rep, 2023, 50: 136. DOI: 10.3892/or.2023.8573. [19] LAI MR, GE YQ, CHEN M, et al. Saikosaponin D inhibits proliferation and promotes apoptosis through activation of MKK4-JNK signaling pathway in pancreatic cancer cells[J]. OncoTargets Ther, 2020, 13: 9465- 9479. DOI: 10.2147/ott.s263322. [20] YANG X, LI SD, LIU JK, et al. Current research status of traditional Chinese medicine in the prevention and treatment of hepatocellular carcinoma by regulating the JAK/STAT signaling pathway[J]. J Clin Hepatol, 2023, 39( 11): 2718- 2729. DOI: 10.3969/j.issn.1001-5256.2023.11.030.杨星, 李淑娣, 刘江凯, 等. 中药调控Janus激酶/信号转导和转录激活因子(JAK/STAT)信号通路防治肝细胞癌的研究现状[J]. 临床肝胆病杂志, 2023, 39( 11): 2718- 2729. DOI: 10.3969/j.issn.1001-5256.2023.11.030. [21] LIU WW, FAN LH, SHAO B, et al. STAT3 promotes migration and invasion of cholangiocarcinoma arising from choledochal cyst by transcriptionally inhibiting miR200c through the c-myb/MEK/ERK signaling pathway[J]. Cell Mol Biol(Noisy-le-grand), 2023, 69( 9): 136- 142. DOI: 10.14715/cmb/2023.69.9.20. [22] WANG YL, ZHANG H, LI G, et al. Study on lymphocyte subsets, interleukin 2, and natural killer cell activity in peripheral blood of patients with liver cancer[J]. Chin J Lab Med, 2004, 27( 12): 844- 845. DOI: 10.3760/j: issn: 1009-9158.2004.12.013.王玉亮, 张珩, 李光, 等. 肝癌患者外周血淋巴细胞亚群、白细胞介素2和自然杀伤细胞活性的研究[J]. 中华检验医学杂志, 2004, 27( 12): 844- 845. DOI: 10.3760/j: issn: 1009-9158.2004.12.013. [23] HUANG TT, HE KX, MAO YY, et al. Genetic variants in PPP2CA are associated with gastric cancer risk in a Chinese population[J]. Sci Rep, 2017, 7: 11499. DOI: 10.1038/s41598-017-12040-z. [24] LV Y, ZHU YF, BAI G. Association between PPP2CA expression and colorectal cancer prognosis tumor marker prognostic study[J]. Int J Surg, 2018, 59: 80- 89. DOI: 10.1016/j.ijsu.2018.09.020. [25] QIAN MX, LU JG, FENG J. Research progress on interleukin-2 and its analogues[J]. Chin J Pharm, 2020, 51( 8): 947- 955. DOI: 10.16522/j.cnki.cjph.2020.08.001.钱梦歆, 路建光, 冯军. 白介素-2及其类似物的研发进展[J]. 中国医药工业杂志, 2020, 51( 8): 947- 955. DOI: 10.16522/j.cnki.cjph.2020.08.001. [26] CALIGIURI A, BECATTI M, PORRO N, et al. Oxidative stress and redox-dependent pathways in cholangiocarcinoma[J]. Antioxidants, 2024, 13( 1): 28. DOI: 10.3390/antiox13010028. [27] BOONSRI B, YACQUB-USMAN K, THINTHARUA P, et al. Effect of combining EGFR tyrosine kinase inhibitors and cytotoxic agents on cholangiocarcinoma cells[J]. Cancer Res Treat, 2021, 53( 2): 457- 470. DOI: 10.4143/crt.2020.585. [28] KIDOIKHAMMOUAN S, LERT-ITTHIPORN W, DEENONPOE R, et al. Targeting EGFR activation to overcome gemcitabine resistance in cholangiocarcinoma[J]. Anticancer Res, 2024, 44( 12): 5393- 5404. DOI: 10.21873/anticanres.17366. [29] PINTER M, PECK-RADOSAVLJEVIC M. Review article: Systemic treatment of hepatocellular carcinoma[J]. Aliment Pharmacol Ther, 2018, 48( 6): 598- 609. DOI: 10.1111/apt.14913. [30] HE JY, HUANG ZR, HAN LZ, et al. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer(Review)[J]. Int J Oncol, 2021, 59( 5): 90. DOI: 10.3892/ijo.2021.5270. [31] PIPER AK, PENNEY C, HOLLIDAY J, et al. EGFR and PI3K signalling pathways as promising targets on circulating tumour cells from patients with metastatic gastric adenocarcinoma[J]. Int J Mol Sci, 2024, 25( 10): 5565. DOI: 10.3390/ijms25105565. -



 PDF下载 ( 63060 KB)
PDF下载 ( 63060 KB)

 下载:
下载: