原发性肝癌患者发生门静脉癌栓的影响因素分析及列线图构建
DOI: 10.12449/JCH251217
Influencing factors for portal vein tumor thrombus in patients with primary hepatic carcinoma and establishment of a nomogram model
-
摘要:
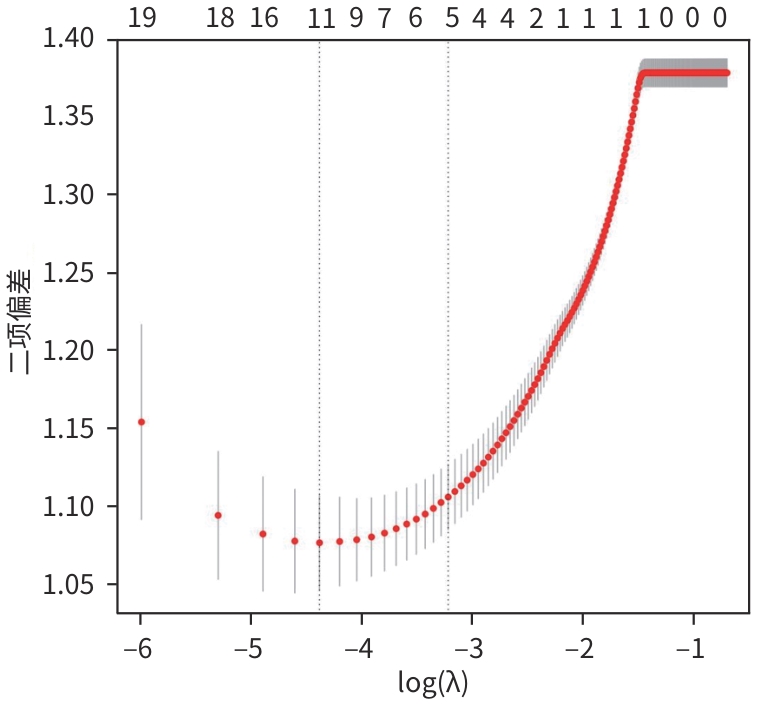
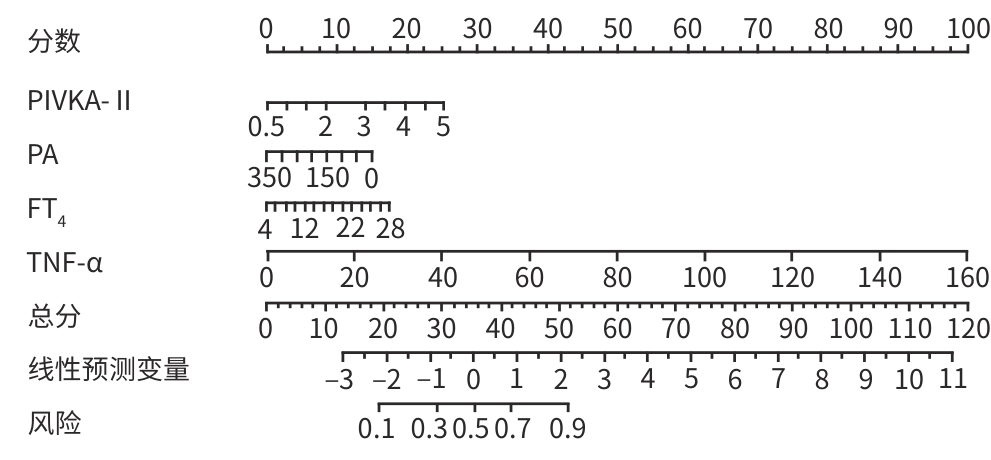
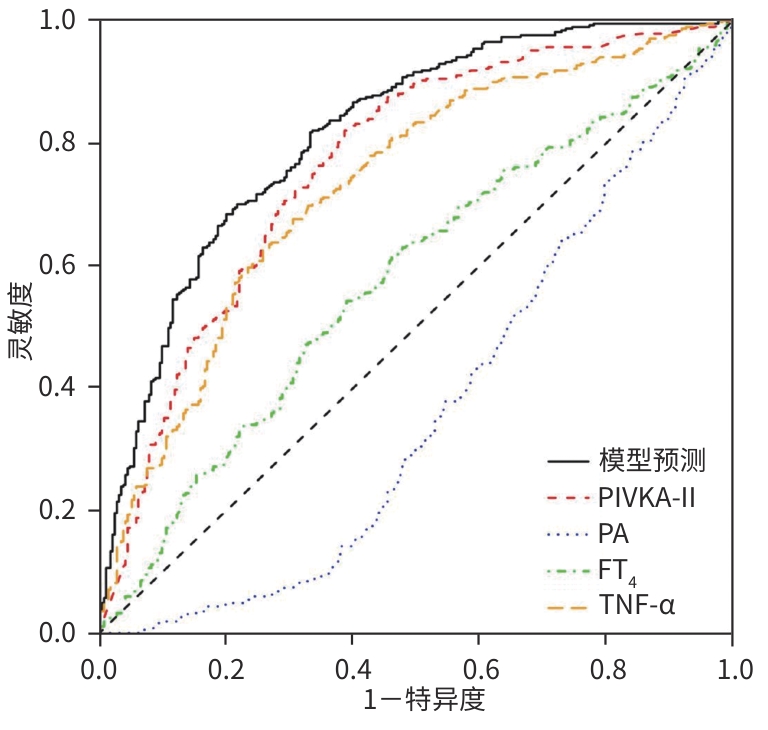
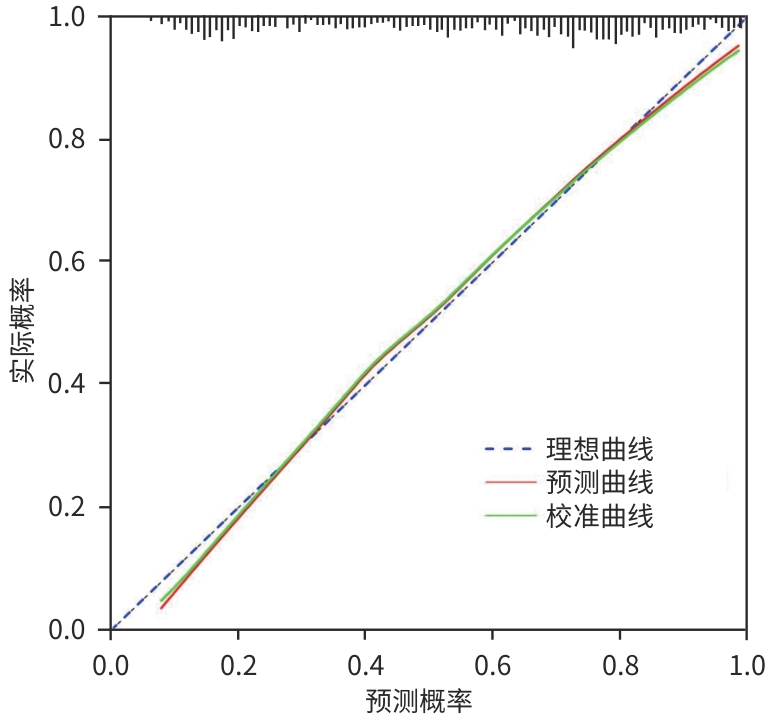
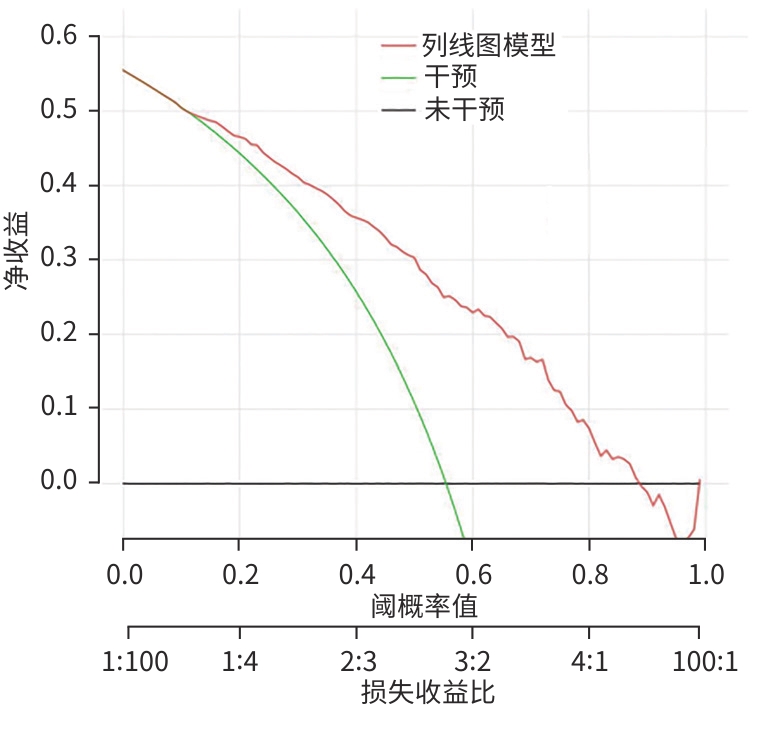
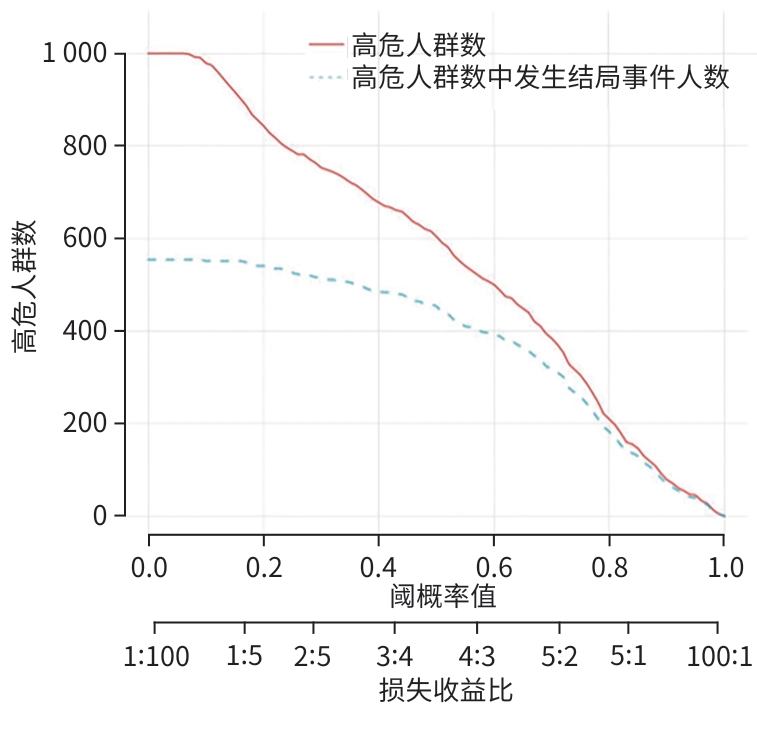
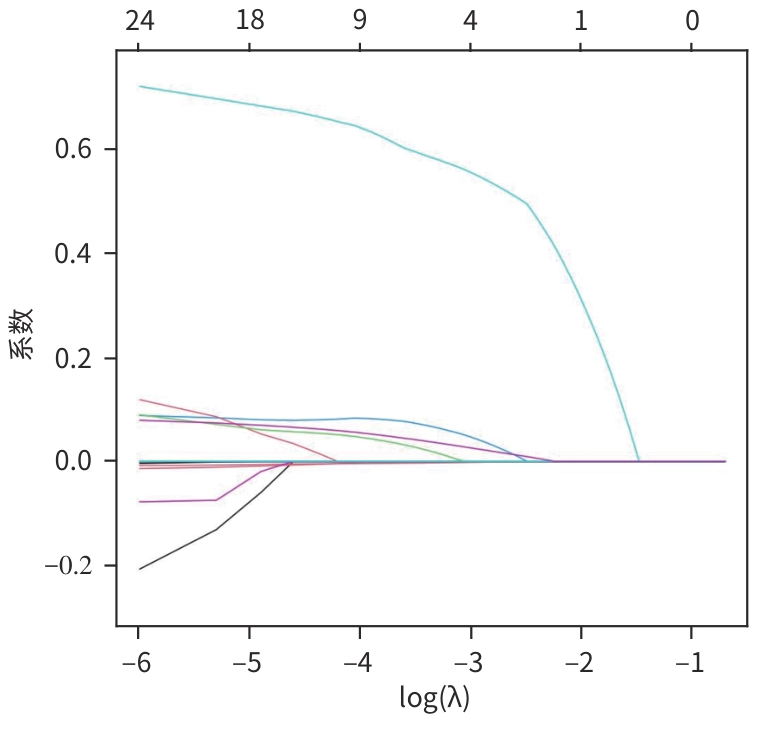
目的 探讨原发性肝癌(PHC)患者发生门静脉癌栓(PVTT)的影响因素,构建预测模型列线图,并对模型性能进行评估。 方法 回顾性纳入2018年1月—2022年12月昆明市第三人民医院收治的664例初诊PHC患者,依据是否发生PVTT分为病例组(n=368)和对照组(n=296)。收集研究对象的一般资料、血生化指标、T淋巴细胞亚群、血常规指标、细胞因子、甲状腺功能指标以及Child-Pugh分级、中国肝癌临床分期(CNLC分期)。符合正态分布的定量资料2组间比较采用成组t检验;非正态分布定量资料2组间比较采用Mann-Whitney U检验。定性资料2组间比较采用χ2检验或Fisher精确检验。将单因素分析中有统计学意义的变量进行Lasso回归,筛选后的变量采用二元Logistic回归分析,确定PHC患者发生PVTT的影响因素。使用“rms”程序包构建列线图;使用“pROC”程序包绘制受试者操作特征曲线(ROC曲线)并计算曲线下面积(AUC);使用“Calibration Curves”程序包绘制校准曲线,使用“rmda”程序包绘制临床决策曲线及临床影响曲线对预测模型进行评价。 结果 PHC患者中发生PVTT 368例(55.42%)。PHC的病因为乙型肝炎的患者有575例(86.60%),其他原因有89例(13.40%),病因以乙型肝炎为主。对照组年龄、前白蛋白(PA)、胆碱酯酶(ChE)、CD3+及CD8+T细胞、癌胚抗原(CEA)、三碘甲状腺原氨酸(T3)、游离三碘甲状腺原氨酸(FT3)水平均高于病例组(P值均<0.05);而病例组Child-Pugh B和C级患者占比、WBC、PLT、AST、ALT、GGT、ALP、异常凝血酶原(PIVKA-Ⅱ)、总胆汁酸(TBA)、超敏C反应蛋白(hs-CRP)、游离甲状腺素(FT4)、甲状腺素(T4)、AFP、IL-6、IL-10、TNF-α水平均高于对照组(P值均<0.05)。Logistic回归分析结果显示,PIVKA-Ⅱ(OR=1.968,95%CI:1.633~2.370,P<0.001)、PA(OR=0.994,95%CI:0.991~0.998,P=0.002)、FT4(OR=1.092,95%CI:1.030~1.159,P=0.003)、TNF-α(OR=1.085,95%CI:1.053~1.119,P<0.001)为PHC患者发生PVTT的独立影响因素,据此建立列线图模型。列线图预测模型的AUC为0.816(95%CI:0.783~0.849),灵敏度为0.834,特异度为0.652。校准曲线显示,此模型预测PHC患者发生PVTT具有较好的一致性,而临床决策曲线、临床影响曲线表示在一定的阈值内此模型具有较好的临床实用性。 结论 PIVKA-Ⅱ、PA、FT4、TNF-α是PHC患者发生PVTT的独立影响因素,联合检测能够较好地预测PHC患者发生PVTT的风险。 Abstract:Objective To investigate the influencing factors for portal vein tumor thrombus (PVTT) in patients with primary liver cancer (PHC), to establish a nomogram predictive model, and to assess the performance of this model. Methods A retrospective analysis was performed for 664 patients with the initial diagnosis of PHC who were admitted to The Third People’s Hospital of Kunming from January 2018 to December 2022, and according to the presence or absence of PVTT, they were divided into case group with 368 patients and control group with 296 patients. Related data were collected from all subjects, including general information, blood biochemical parameters, T lymphocyte subsets, routine blood test results, cytokines, thyroid function parameters, Child-Pugh score, and China Liver Cancer Staging (CNLC) stage. The t-test was used for comparison of normally distributed quantitative data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed quantitative data between two groups; the chi-square test or the Fisher’s exact test was used for comparison of qualitative data between two groups. The Lasso regression analysis was performed for the variables with statistical significance in the univariate analysis, and the binary logistic regression analysis was performed for the screened variables to determine the influencing factors for PVTT in patients with PHC. The “rms” package was used to establish a nomogram model; the “pROC” package was used to plot the receiver operating characteristic (ROC) curve and calculate the area under the ROC curve (AUC); the “Calibration Curves” package was used to plot calibration curves, and the “rmda” package was used to plot clinical decision curves and clinical impact curves for the assessment of the predictive model. Results Among the 664 patients with PHC, 368 (55.42%) developed PVTT. As for the etiology of PHC, there were 575 patients with hepatitis B (86.60%) and 89 with other causes (13.40%), with hepatitis B as the main cause of PHC. Compared with the case group, the control group had significantly higher age, prealbumin (PA), cholinesterase, CD3+T cells, CD8+T cells, carcinoembryonic antigen, triiodothyronine, and free triiodothyronine (all P<0.05); compared with the control group, the case group had a significantly higher proportion of patients with Child-Pugh class B/C PHC and significantly higher levels of white blood cell count, platelet count, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, alkaline phosphatase, abnormal prothrombin (PIVKA-Ⅱ), total bile acid, high-sensitivity C-reactive protein, free thyroxine (FT4), thyroxine, alpha-fetoprotein, interleukin-6, interleukin-10, and tumor necrosis factor-α (TNF-α) (all P<0.05). The logistic regression analysis showed that PIVKA-Ⅱ (odds ratio [OR]=1.968, 95% confidence interval [CI]: 1.633 — 2.370, P<0.001), PA (OR=0.994, 95% CI: 0.991 — 0.998, P=0.002), FT4 (OR=1.092, 95% CI: 1.030 — 1.159, P=0.003), and TNF-α (OR=1.085, 95% CI: 1.053 — 1.119, P<0.001) were independent influencing factors for PVTT in patients with PHC, and a nomogram model was established based on these factors. The nomogram model had an AUC of 0.816 (95% CI: 0.783 — 0.849), a sensitivity of 0.834, and a specificity of 0.652. The calibration curve showed that this model had good consistency in predicting the onset of PVTT in patients with PHC, while the clinical decision curve and the clinical impact curve showed that this model had good clinical practicability within a certain threshold. Conclusion PIVKA-Ⅱ, PA, FT4, and TNF-α are independent influencing factors for the onset of PVTT in patients with PHC, and combined measurement of these four indicators can effectively predict the risk of PVTT in patients with PHC. -
Key words:
- Liver Neoplasms /
- Portal Vein Tumor Thrombus /
- Risk Factors /
- Nomograms
-
表 1 两组患者临床基线特征比较
Table 1. Comparison of clinical baseline characteristics between the two groups of patients
指标 对照组(n=296) 病例组(n=368) 统计值 P值 年龄(岁) 55.00(50.00~63.00) 53.00(48.00~60.00) Z=-2.361 0.018 BMI(kg/m2) 22.42(20.07~25.10) 21.81(19.93~24.25) Z=-1.792 0.073 Child-Pugh分级[例(%)] χ2=22.804 < 0.001 A级 133(44.93) 101(27.44) B级 105(35.47) 160(43.48) C级 58(19.59) 107(29.08) CNLC分期[例(%)] χ2=0.349 0.608 Ⅰ期/Ⅱ期 150(50.68) 178(48.37) Ⅲ期/Ⅳ期 146(49.32) 190(51.63) 性别[例(%)] χ2=0.132 0.776 男 153(51.69) 185(50.27) 女 143(48.31) 183(49.73) 乙型肝炎家族史[例(%)] χ2=1.777 0.249 无 285(96.28) 346(94.02) 有 11(3.72) 22(5.98) 饮酒史[例(%)] χ2=0.251 0.616 无 157(53.04) 188(51.09) 有 139(46.96) 180(48.91) 吸烟史[例(%)] χ2=0.634 0.473 无 138(46.62) 183(49.73) 有 158(53.38) 185(50.27) 吸毒史[例(%)] χ2=3.594 0.079 无 277(93.58) 329(89.40) 有 19(6.42) 39(10.60) 病因[例(%)] χ2=0.709 0.400 乙型肝炎 260(87.84) 315(85.60) 其他 36(12.16) 53(14.40) 表 2 两组患者实验室检查指标比较
Table 2. Comparison of laboratory test indicators between two groups of patients
指标 对照组(n=296) 病例组(n=368) 统计值 P值 血常规及凝血功能指标 WBC(×109/L) 5.06(3.66~6.62) 5.70(4.04~7.96) Z=-3.364 <0.001 Hb(g/L) 133.50(111.75~152.00) 129.00(108.75~149.00) Z=-1.812 0.070 PLT(×109/L) 101.00(68.00~164.25) 122.50(83.75~194.50) Z=-3.544 <0.001 FIB(g/L) 2.75(1.99~3.85) 2.95(2.15~4.07) Z=-1.841 0.066 血生化指标 PIVKA-Ⅱ(lg mAU/mL) 2.14(1.48~3.57) 3.94(3.03~4.52) Z=-1.618 <0.001 AST(U/L) 51.00(33.00~90.00) 94.00(57.00~171.25) Z=-8.832 <0.001 ALT(U/L) 37.00(22.75~59.25) 45.00(30.00~81.00) Z=-3.994 <0.001 TP(g/L) 65.56 ± 8.11 64.86±8.58 t=-1.432 0.278 PA(mg/L) 113.65(77.65~177.52) 92.85(66.47~120.75) Z=-5.973 <0.001 GGT(U/L) 87.00(49.00~188.25) 216.00(117.75~371.00) Z=-9.549 <0.001 ALP(U/L) 156.50(115.75~232.25) 213.50(143.00~331.00) Z=-5.976 <0.001 ChE(U/L) 3 934.50(2 461.50~6 154.00) 3 167.00(2 125.00~4 457.75) Z=-5.080 <0.001 TBA(μmol/L) 17.95(7.52~48.47) 25.40(10.20~61.68) Z=-2.857 0.004 TG(mmol/L) 0.92(0.62~1.28) 0.89(0.65~1.26) Z=-0.556 0.579 TC(mmol/L) 3.82(3.13~4.91) 3.78(3.00~4.75) Z=-0.646 0.518 LDL(mmol/L) 2.38(1.77~3.13) 2.48(1.82~3.33) Z=-1.206 0.228 Cr(μmol/L) 65.00(55.75~79.25) 66.00(57.00~81.00) Z=-0.829 0.407 UA(μmol/L) 317.50(256.75~409.25) 331.00(250.00~414.00) Z=-0.103 0.918 BG(mmol/L) 5.46(4.92~6.33) 5.40(4.77~6.20) Z=-1.375 0.169 hs-CRP(mg/L) 9.56(1.92~26.92) 24.54(9.18~49.92) Z=-7.205 <0.001 AFP(lg µg/L) 1.22(0.61~2.67) 2.90(1.33~4.55) Z=-8.805 <0.001 CEA(µg/L) 3.30(2.30~5.07) 3.06(1.89~4.70) Z=-2.091 0.037 甲状腺功能指标 TSH(mIU/mL) 2.34(1.59~3.65) 2.34(1.59~3.32) Z=-0.947 0.344 T3(nmol/L) 1.59(1.23~1.90) 1.39(1.10~1.76) Z=-3.879 <0.001 T4(nmol/L) 99.38(85.06~116.53) 106.90(88.40~128.85) Z=-3.169 0.002 FT3(pmol/L) 3.91(3.11~4.57) 3.44(2.78~4.11) Z=-4.842 <0.001 FT4(pmol/L) 16.09(14.43~18.08) 16.91(15.13~18.91) Z=-3.394 <0.001 T淋巴细胞计数 CD3+(个/μL) 780.21(593.03~1 041.05) 729.42(573.33~924.39) Z=-2.229 0.026 CD4+(个/μL) 440.92(316.78~569.59) 416.21(307.06~547.04) Z=-1.325 0.185 CD8+(个/μL) 276.00(184.81~431.90) 260.40(168.04~360.96) Z=-2.309 0.021 细胞因子 IL-6(pg/mL) 26.99(10.85~53.88) 41.89(23.09~81.07) Z=-5.902 <0.001 IL-10(pg/mL) 4.57(3.34~7.18) 5.44(3.95~8.82) Z=-4.002 <0.001 TNF-α(pg/mL) 1.97(1.46~3.45) 4.35(2.29~9.05) Z=-10.174 <0.001 表 3 PHC患者发生PVTT的二元Logistic回归分析结果
Table 3. Results of binary Logistic regression analysis of PVTT in PHC patients
指标 β值 SE Wald OR 95%CI P值 PIVKA-Ⅱ(lg mAU/mL) 0.677 0.095 50.746 1.968 1.633~2.370 <0.001 PA(mg/L) -0.006 0.002 10.076 0.994 0.991~0.998 0.002 FT4(pmol/L) 0.088 0.030 8.540 1.092 1.030~1.159 0.003 TNF-α(pg/mL) 0.082 0.016 27.448 1.085 1.053~1.119 <0.001 常量 -3.483 0.624 31.175 0.031 <0.001 表 4 各变量及预测模型评估PHC发生PVTT的ROC曲线分析
Table 4. ROC analysis of each variable and predictive model in assessing PVTT in patients with PHC
指标 AUC 95%CI Cut-off值 灵敏度 特异度 P值 PIVKA-Ⅱ(lg mAU/mL) 0.762 0.725~0.799 2.633 0.829 0.605 <0.001 PA(mg/L) 0.365 0.322~0.409 147.100 0.101 0.632 <0.001 FT4(pmol/L) 0.577 0.533~0.620 16.301 0.620 0.537 <0.001 TNF-α(pg/mL) 0.729 0.691~0.768 2.830 0.677 0.693 <0.001 预测模型 0.816 0.783~0.849 0.834 0.652 <0.001 -
[1] LI Z, ZHU JY. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707.李照, 朱继业.《原发性肝癌诊疗指南(2024年版)》解读[J]. 临床肝胆病杂志, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707. [2] YUAN C. Predictive nomogram model for hepatocellular carcinoma complicated with portal vein tumor thrombus[D]. Nanchang: Nanchang University, 2023.袁诚. 肝细胞癌合并门静脉癌栓的预测列线图模型[D]. 南昌: 南昌大学, 2023. [3] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [4] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [5] PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. [6] XIAO TT, DENG T. Progress in the treatment of hepatocellular carcinoma complicated with portal vein tumor thrombosis[J]. Anhui Med Pharm J, 2025, 29( 3): 460- 466. DOI: 10.3969/j.issn.1009-6469.2025.03.008.肖婷婷, 邓坦. 原发性肝癌合并门静脉癌栓治疗进展[J]. 安徽医药, 2025, 29( 3): 460- 466. DOI: 10.3969/j.issn.1009-6469.2025.03.008. [7] HUANG JJ. Analysis of clinical characteristics of primary liver cancer complicated with portal vein tumor thrombus[D]. Chagnchun: Jilin University, 2023.黄剑洁. 原发性肝癌合并门静脉癌栓的临床特征分析[D]. 长春: 吉林大学, 2023. [8] WANG JC, XIA AL, XU Y, et al. Comprehensive treatments for hepatocellular carcinoma with portal vein tumor thrombosis[J]. J Cell Physiol, 2019, 234( 2): 1062- 1070. DOI: 10.1002/jcp.27324. [9] WANG W. Analysis of risk factors for primary liver cancer complicated with portal vein tumor thrombus in high-altitude areas[D]. Xining: Qinghai University, 2023.王雯. 高海拔地区原发性肝癌合并门静脉癌栓的危险因素分析[D]. 西宁: 青海大学, 2023. [10] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [11] Liver Cancer Professional Committee of the Chinese Medical Doctor Association. Diagnosis and treatment guidelines for hepatocellular carcinoma complicated with portal vein tumor thrombus in China(2021 edition)[J]. Chin Med J, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn11-2137-20211117-02567.中国医师协会肝癌专业委员会. 中国肝细胞癌合并门静脉癌栓诊疗指南(2021年版)[J]. 中华医学杂志, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn112137-20211117-02567. [12] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [13] TONG JS, MA ZP, MAO SQ, et al. Prediction value of PiVKA-Ⅱ for hepatocellular carcinoma complicated with portal vein tumor thrombosis[J]. J Hepatopancreatobiliary Surg, 2022, 34( 1): 23- 29. DOI: 10.11952/j.issn.1007-1954.2022.01.006.童敬澍, 马浙平, 毛书奇, 等. 异常凝血酶原对肝癌合并门静脉癌栓的预测价值[J]. 肝胆胰外科杂志, 2022, 34( 1): 23- 29. DOI: 10.11952/j.issn.1007-1954.2022.01.006. [14] DU CX, LI DR, ZHANG SB, et al. Progress and strategies of surgical treatment of hepatocellular carcinoma with portal veintumor thrombus[J]. Chin J Dig Surg, 2023, 22( 2): 214- 218. DOI: 10.3760/cma.j.cn115-610-20230209-00054.杜成旭, 李冬瑞, 张树彬, 等. 肝癌合并门静脉癌栓外科治疗进展及策略[J]. 中华消化外科杂志, 2023, 22( 2): 214- 218. DOI: 10.3760/cma.j.cn1-15610-20230209-00054. [15] ZHANG DX, ZHANG YH, GUI RH, et al. Diagnostic value of α-fetoprotein, α-fetoprotein heterogenes and tumor necrosis factor-α in the diagnosis of primary liver cancer[J]. China Mod Med, 2021, 28( 26): 223- 225, 229. DOI: 10.3969/j.issn.1674-4721.2021.26.061.张丹霞, 张永虎, 桂若虎, 等. 甲胎蛋白、甲胎蛋白异质体与肿瘤坏死因子α检测在原发性肝癌诊断中的应用价值[J]. 中国当代医药, 2021, 28( 26): 223- 225, 229. DOI: 10.3969/j.issn.1674-4721.2021.26.061. [16] YE S. The value of TNF-α and hs-CRP levels in peripheral blood in prognosis evaluation of patients with liver cancer[J]. Chin J Public Health Eng, 2019, 18( 5): 740- 742. DOI: 10.19937/j.issn.1671-4199.2019.05.037.叶赛. 外周血TNF-α和hs-CRP水平在肝癌患者预后评估中的价值[J]. 中国卫生工程学, 2019, 18( 5): 740- 742. DOI: 10.19937/j.issn.1671-4199.2019.05.037. [17] REN XJ. The effect and efficacy of ulinastatin on TNF-α and hs-CRP level of hepatocellular carcinoma patients with septic shock after surgery[J]. Pract J Cancer, 2017, 32( 12): 1937- 1939. DOI: 10.3969/j.issn.1001-5930.2017.12.007.任宪军. 乌司他丁对肝癌术后感染性休克患者TNF-α、hs-CRP水平的影响及疗效评估[J]. 实用癌症杂志, 2017, 32( 12): 1937- 1939. DOI: 10.3969/j.issn.1001-5930.2017.12.007. [18] ZHANG P, MENG XY. Expression of GRP-78 and COX-2 in hepatocellular carcinoma and their correlation with clinicopathological features[J]. Chin J Public Health Eng, 2019, 18( 1): 76- 78. DOI: 10.19937/j.issn.1671-4199.2019.01.028.张萍, 孟宪宇. 肝癌组织中GRP-78和COX-2表达及与临床病理特征的相关性[J]. 中国卫生工程学, 2019, 18( 1): 76- 78. DOI: 10.19937/j.issn.1671-4199.2019.01.028. [19] LI YY, LIU HC, SONG YS, et al. Application and clinical significance of detection of alpha-fetoprotein, alpha-fetoprotein heterogeneity and TNF-α in diagnosis of primary liver cancer[J]. Heilongjiang J Tradit Chin Med, 2021, 50( 1): 24- 25.李莹莹, 刘红春, 宋银森, 等. 原发性肝癌诊断中甲胎蛋白、甲胎蛋白异质体、TNF-α检测的应用及临床意义分析[J]. 黑龙江中医药, 2021, 50( 1): 24- 25. [20] YUAN L, SHEN SQ, LU X. An evaluation of prediction value of immune inflammatory factor on prognosis in patients with hepatocellular carcinoma[J]. Chin Youjiang Med J, 2016, 44( 1): 14- 18. DOI: 10.3969/j.issn.1003-1383.2016.01.004.袁林, 沈世强, 卢欣. 外周血免疫炎症因子对肝细胞肝癌预后预测作用的评价[J]. 右江医学, 2016, 44( 1): 14- 18. DOI: 10.3969/j.issn.1003-1383.2016.01.004. [21] LI SQ, ZHANG HQ. Research progress in liver damage related to thyroid dysfunction[J]. Chin J Pract Intern Med, 2022, 42( 2): 164- 167. DOI: 10.19538/j.nk2022020116.李舒祺, 张海清. 甲状腺功能异常相关性肝损害研究进展[J]. 中国实用内科杂志, 2022, 42( 2): 164- 167. DOI: 10.19538/j.nk2022020116. [22] GUO ZP, WANG CF, LU JX, et al. Correlations between thyroid hormone levels and severity of condition in patients with hepatitis B cirrhosis[J]. Henan Med Res, 2020, 29( 21): 3855- 3858. DOI: 10.3969/j.issn.1004-437X.2020.21.005.郭志鹏, 王春峰, 陆佳欣, 等. 乙肝肝硬化患者甲状腺激素水平与病情严重程度的相关性[J]. 河南医学研究, 2020, 29( 21): 3855- 3858. DOI: 10.3969/j.issn.1004-437X.2020.21.005. [23] SONG SJ, SU GH. Application of serum total bile acid, cholinesterase and prealbumin detection in the diagnosis of liver disease[J]. J Med Inf, 2021, 34( 23): 9- 11.宋少娟, 苏国华. 血清总胆汁酸、胆碱酯酶和前白蛋白检测在肝病诊断中应用探析[J]. 医学信息, 2021, 34( 23): 9- 11. [24] LI X, ZHANG PA. Study on application of serum total bile acid and enzymatic indices in differential diagnosis of patients with liver diseases[J]. J Clin Exp Med, 2015, 14( 8): 658- 660. DOI: 10.3969/j.issn.1671-4695.2015.08.018.李欣, 张平安. 血清总胆汁酸及酶学指标在鉴别诊断肝病患者中的应用分析[J]. 临床和实验医学杂志, 2015, 14( 8): 658- 660. DOI: 10.3969/j.issn.1671-4695.2015.08.018. [25] LIAO GP, MO MR, ZHANG L, et al. Diagnostic value of total bile acids and total cholesterol in serum for diagnosis of liver diseases[J]. Mod Diagn Treat, 2016, 27( 16): 2965- 2967.廖国平, 莫敏如, 张蕾, 等. 总胆汁酸与血清总胆固醇检测对肝病诊断的价值分析[J]. 现代诊断与治疗, 2016, 27( 16): 2965- 2967. [26] WENG LY. Correlation between serum PA, GGT and CHE expression levels and liver function in patients with liver cirrhosis[J]. Mod Diagn Treat, 2021, 32( 2): 262- 263.翁丽燕. 肝硬化患者血清PA、GGT、CHE表达水平与肝功能的相关性[J]. 现代诊断与治疗, 2021, 32( 2): 262- 263. [27] LI HL, ZHANG DJ, SUN ZJ. Changes and clinical significance of serum prealbumin, apolipoprotein-A1, cholinesterase, γ-glutamyltranspeptidase, prothrombin time and total bile acid in patients with liver disease[J]. Chin J Infect Dis, 2016, 34( 3): 182- 185. DOI: 10.3760/cma.j.issn.1000-6680.2016.03.011.李宏良, 张东军, 孙志坚. 肝病患者血清前白蛋白、载脂蛋白-A1、胆碱酯酶、γ-谷氨酰转肽酶、凝血酶原时间、总胆汁酸的变化及临床意义[J]. 中华传染病杂志, 2016, 34( 3): 182- 185. DOI: 10.3760/cma.j.issn.1000-6680.2016.03.011. [28] ZHANG YH, HU T, WANG Z, et al. Value of serum prealbumin, total bilirubin, and prothrombin activity in predicting liver injury caused by targeted drugs combined with transcatheter arterial chemoembolization in treatment of primary liver cancer[J]. Clin J Med Offic, 2024, 52( 7): 706- 708. DOI: 10.16680/j.1671-3826.2024.07.12.张昀昊, 胡涛, 王钊, 等. 血清前白蛋白、总胆红素、凝血酶原活动度对原发性肝癌靶向药物联合经动脉插管化疗栓塞术治疗所致肝损伤预测价值分析[J]. 临床军医杂志, 2024, 52( 7): 706- 708. DOI: 10.16680/j.1671-3826.2024.07.12. [29] LYU ML, ZHONG X, HU R, et al. Relationship between serum prealbumin and peripheral blood lymphocyte levels in patients with primary liver cancer[J]. Mod Med J China, 2023, 25( 2): 9- 14.吕敏玲, 钟欣, 胡锐, 等. 原发性肝癌患者血清前白蛋白与外周血淋巴细胞水平的关系[J]. 中国现代医药杂志, 2023, 25( 2): 9- 14. [30] ZU HL, WANG HL, LI CF, et al. Preoperative prealbumin levels on admission as an independent predictive factor in patients with gastric cancer[J]. Medicine, 2020, 99( 11): e19196. DOI: 10.1097/md.000000000-0019196. [31] LIU ZC, JIA WP, LYU HJ. Application of combined detection of serum albumin, tumor-specific growth factor and serum alpha-fetoprotein in primary liver cancer[J]. Chronic Pathematology J, 2023, 24( 4): 611- 613.刘志超, 贾伟萍, 吕豪杰. 血清白蛋白和肿瘤特异性生长因子及血清甲胎蛋白联合检测在原发性肝癌中的应用[J]. 慢性病学杂志, 2023, 24( 4): 611- 613. -



 PDF下载 ( 2863 KB)
PDF下载 ( 2863 KB)

 下载:
下载: