扶正化瘀胶囊对代谢相关脂肪性肝病肝纤维化小鼠模型疤痕相关巨噬细胞的调控作用及机制分析
DOI: 10.12449/JCH251215
Regulatory role and mechanism of Fuzheng Huayu Capsule on scar-associated macrophages in a mouse model of liver fibrosis in metabolic associated fatty liver disease
-
摘要:
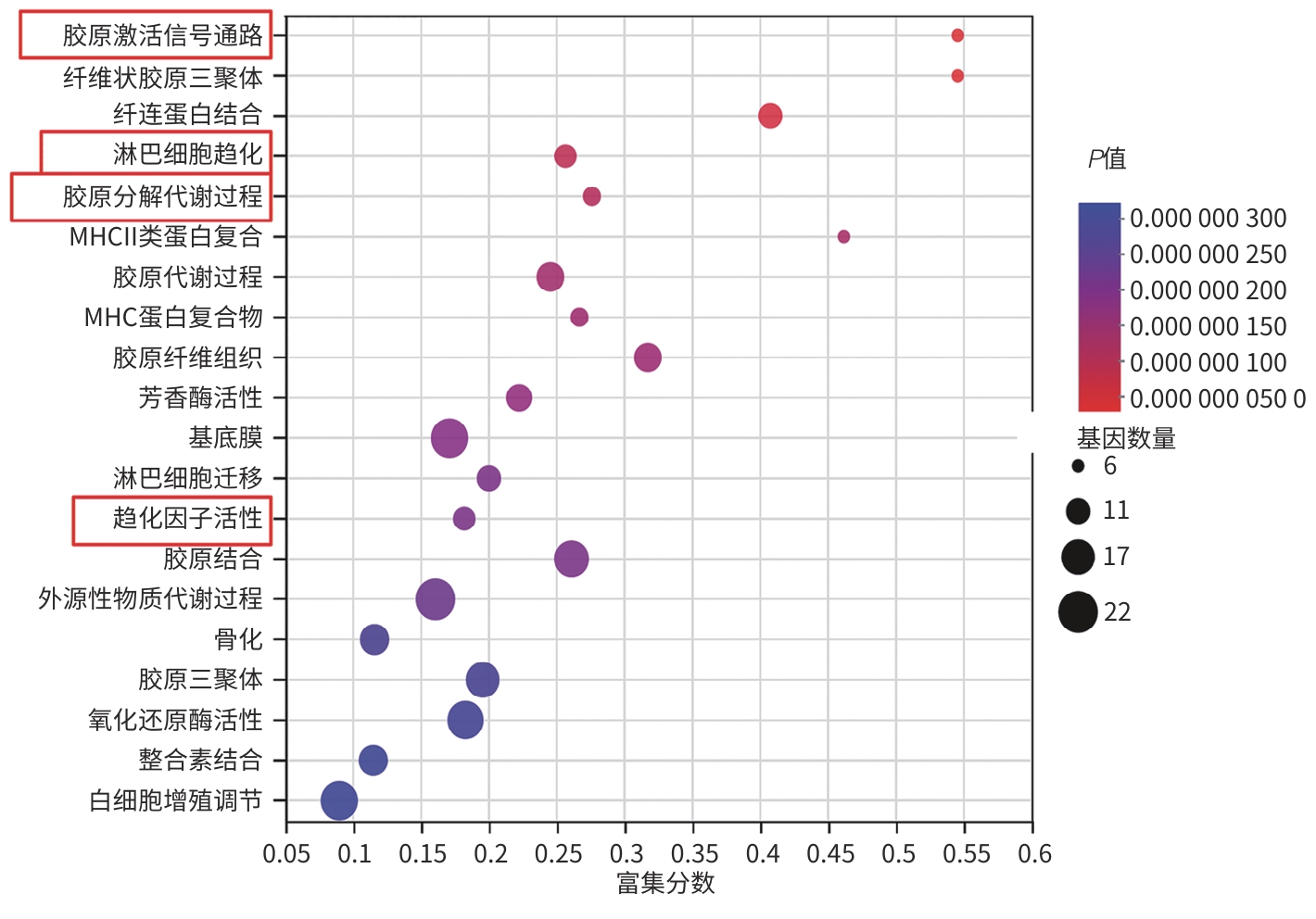
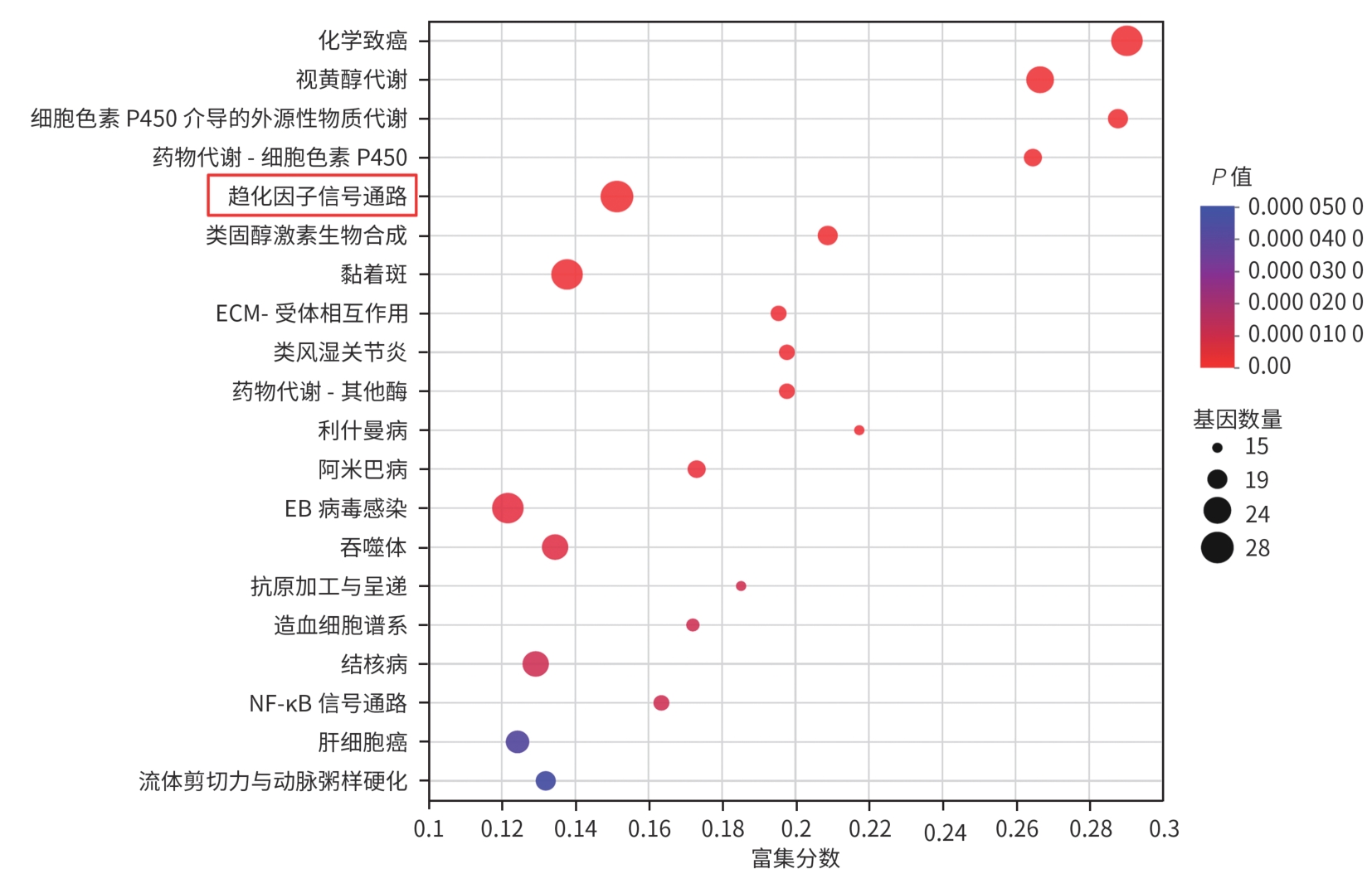
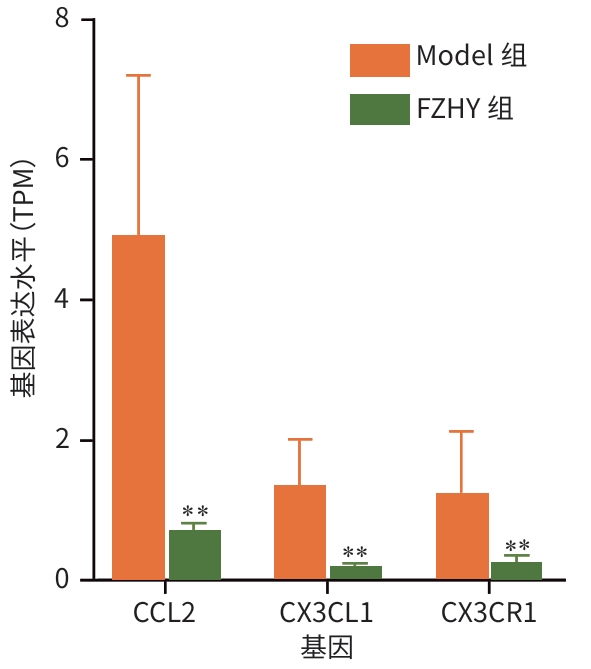
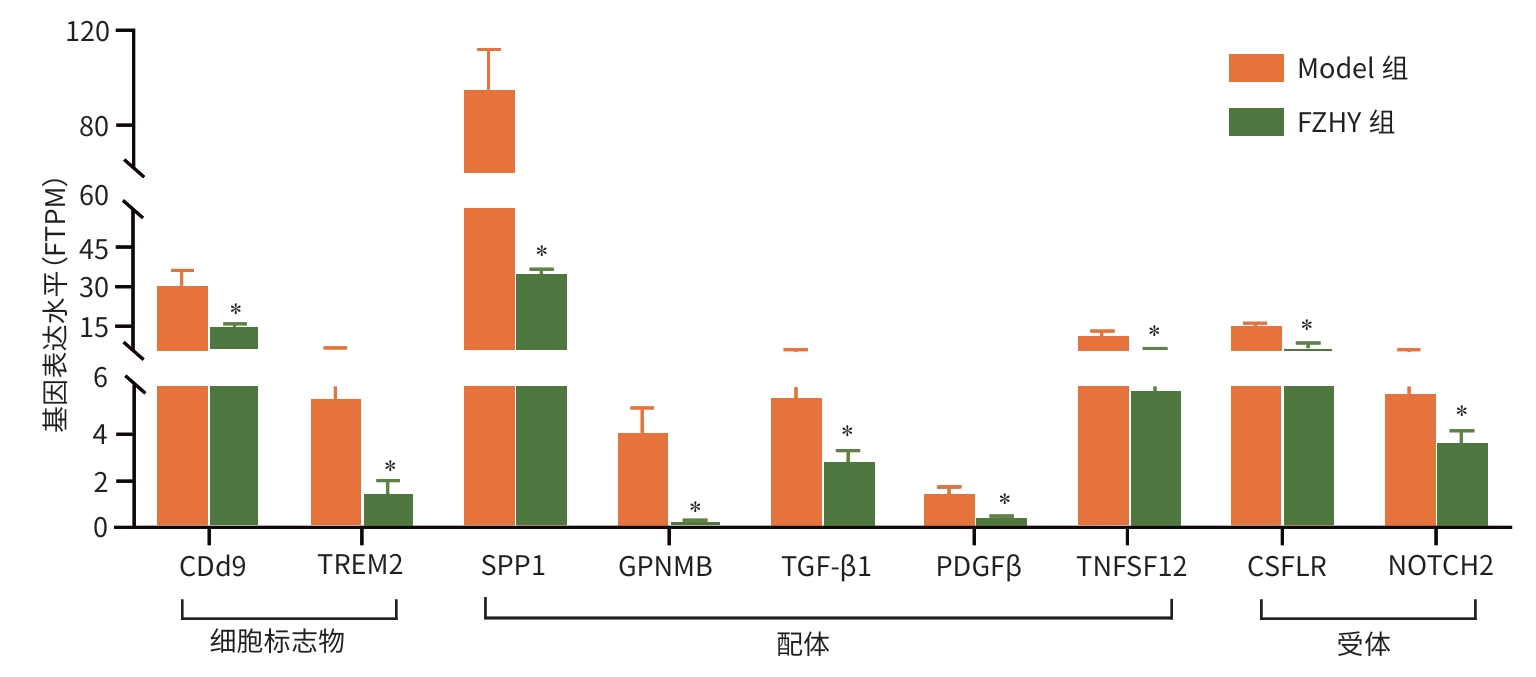
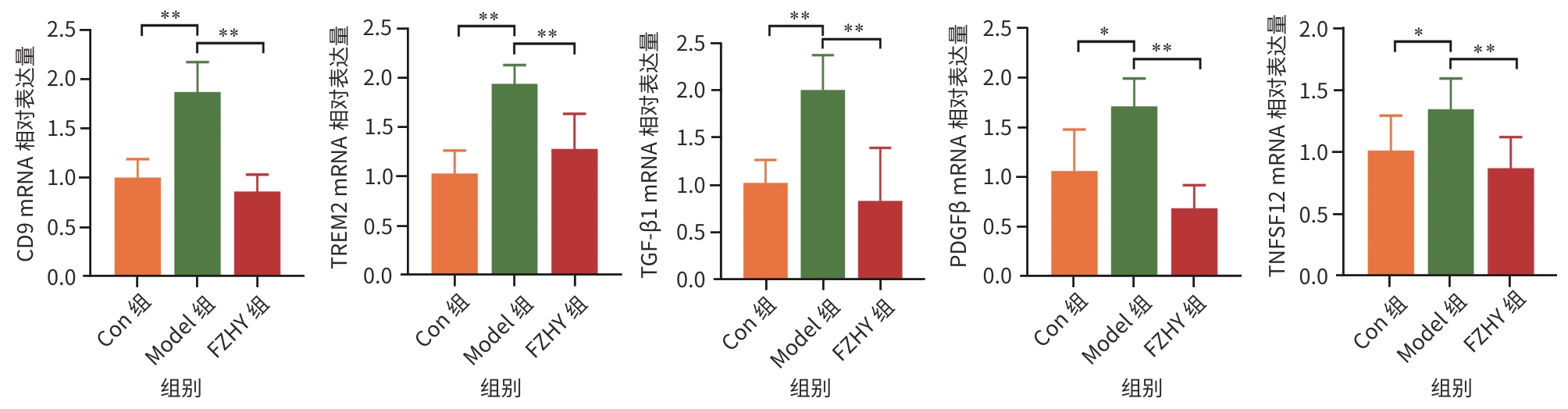
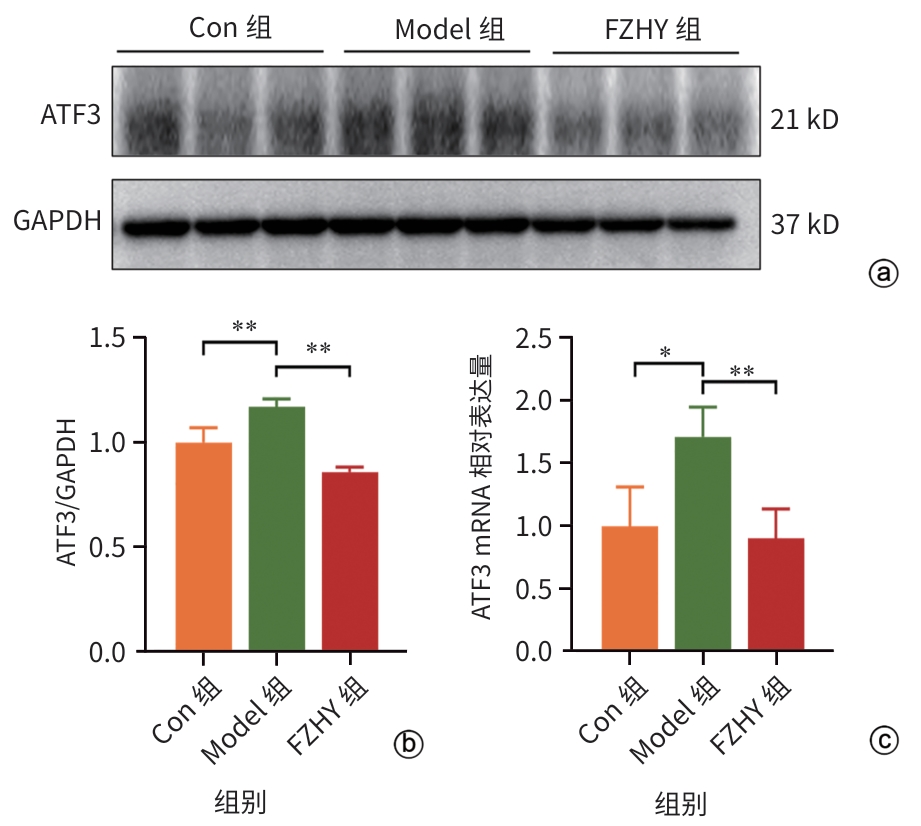
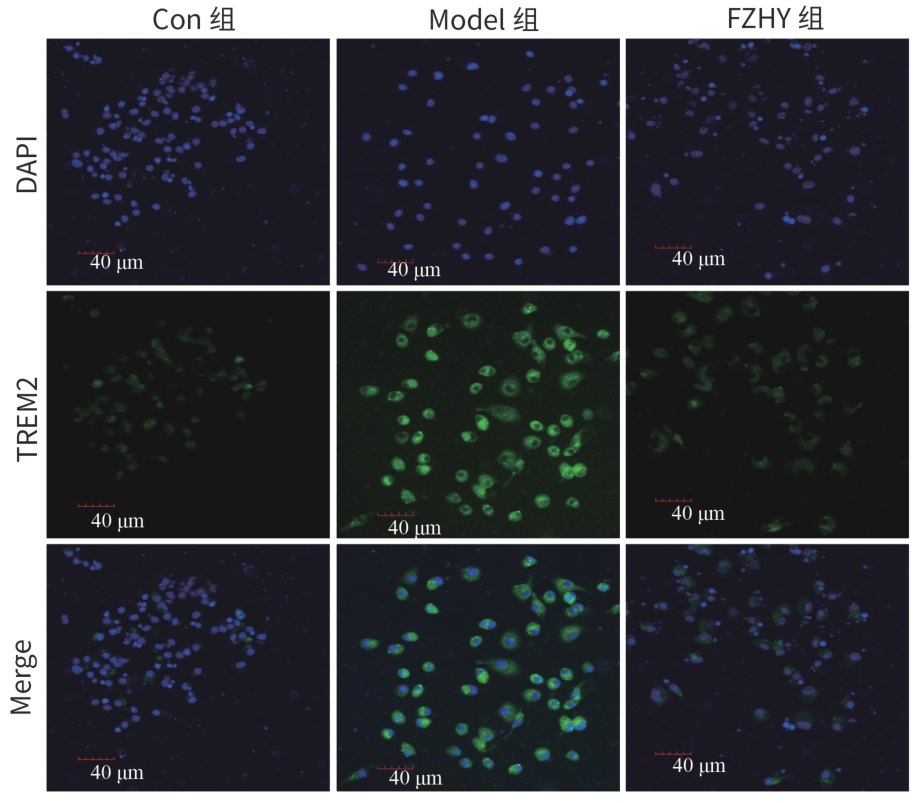
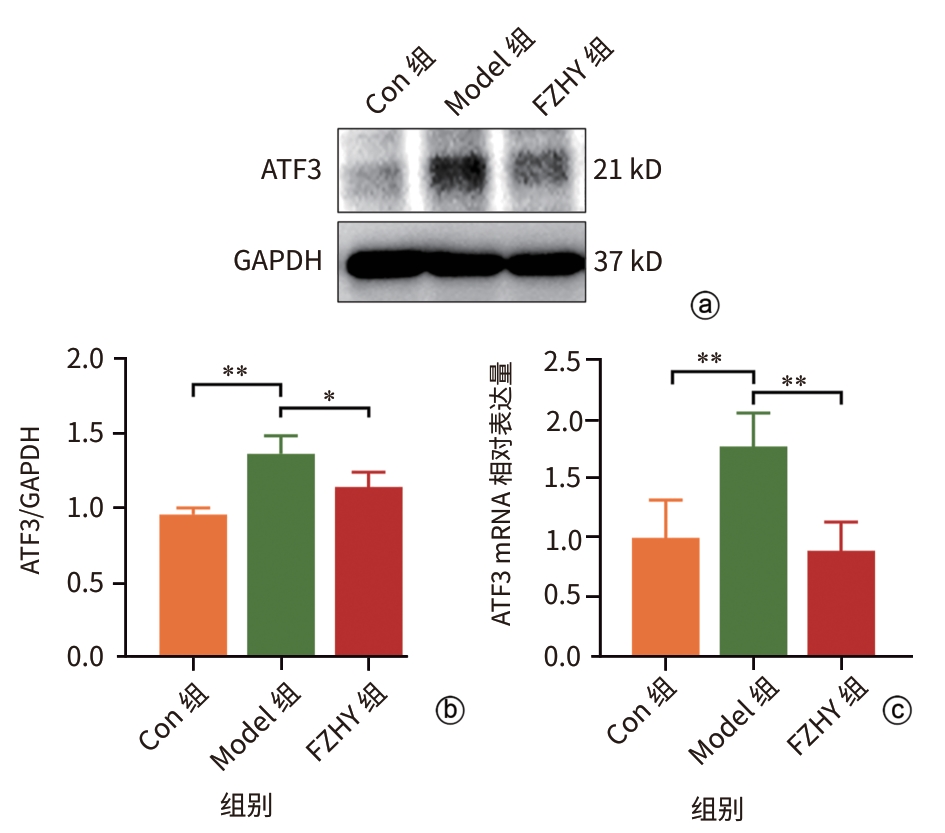
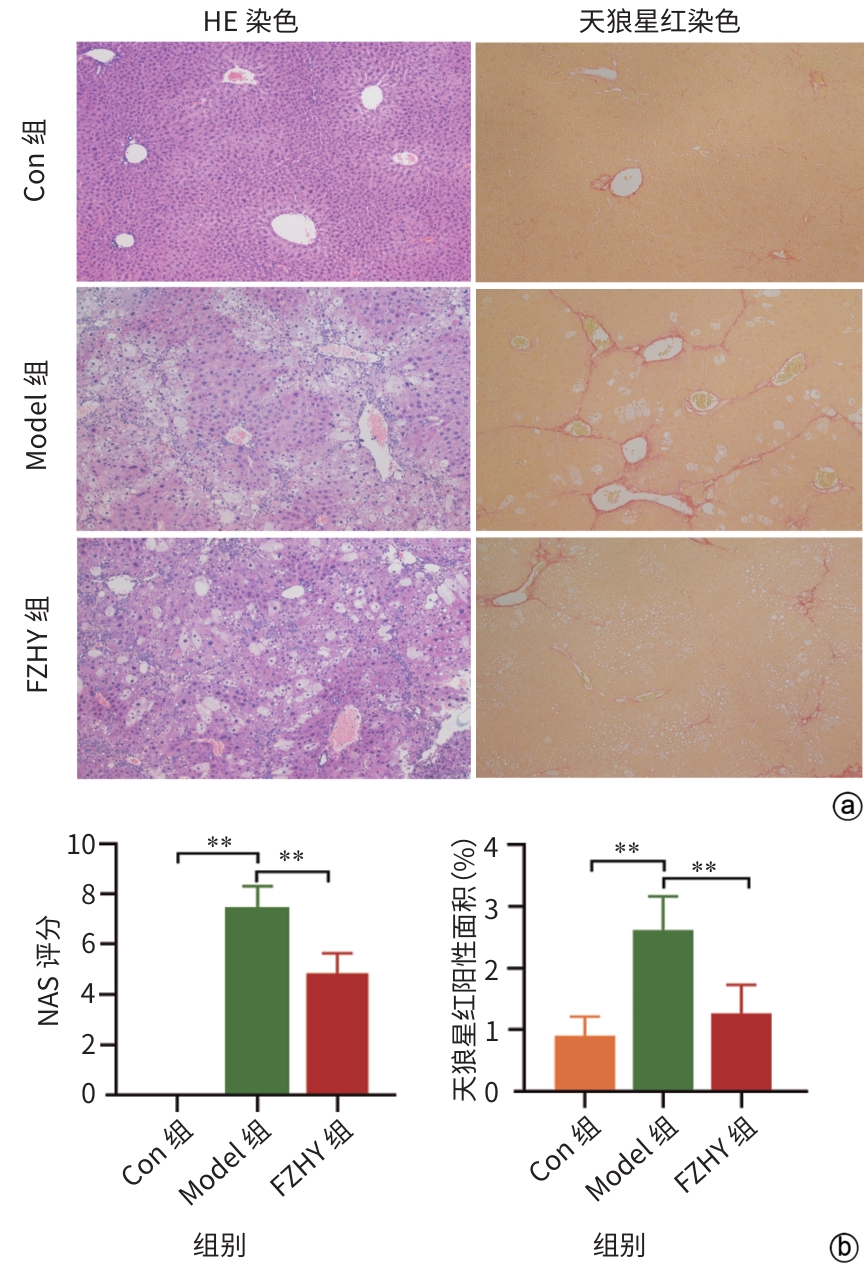
目的 从调控疤痕相关巨噬细胞(SAM)角度探讨扶正化瘀胶囊改善代谢相关脂肪性肝病肝纤维化的分子机制。 方法 24只C57小鼠随机分为对照组(Con组)、模型组(Model组)和扶正化瘀胶囊组(FZHY组)。采用高脂饮食(HFD)联合CCl4腹腔注射6周构建代谢相关脂肪性肝病肝纤维化小鼠模型。自造模第2周起,连续灌胃5周,FZHY组小鼠给予扶正化瘀胶囊灌胃,Con组和Model组以等量生理盐水灌胃。检测血清肝酶、肝脏甘油三酯(TG)及羟脯氨酸(Hyp)含量。观察肝组织HE及天狼星红染色情况。取Model组和FZHY组肝组织样本各3例,进行转录组测序探究扶正化瘀胶囊改善代谢相关脂肪性肝病肝纤维化的分子机制。Western Blot和RT-qPCR检测肝组织中SAM 标志物白细胞分化抗原9(CD9)和髓系细胞触发受体2(TREM2)、SAM促纤维化功能基因包括转化生长因子-β1(TGF-β1)、血小板源性生长因子β(PDGFβ)、TNF超家族成员12(TNFSF12)及上游调控分子转录激活因子3(ATF3)的蛋白/基因表达。采用扶正化瘀胶囊含药血清干预脂多糖(LPS)联合TGF-β1诱导的促炎骨髓来源巨噬细胞(BMDM),免疫荧光、Western Blot和RT-qPCR检测TREM2和ATF3的表达。计量资料两组间比较采用成组t检验,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 FZHY组小鼠肝脏肝酶、肝组织TG和Hyp含量、NAS评分以及天狼星红染色阳性面积较Model组显著降低(P值均<0.01)。RNA-seq分析显示差异基因主要富集于趋化因子信号通路,扶正化瘀胶囊能够显著下调C-C基元趋化因子配体2、CX3C趋化因子配体1及其受体CX3C趋化因子受体1的表达(P值均<0.01)。扶正化瘀胶囊显著降低小鼠肝组织CD9和TREM2,以及ATF3的蛋白及基因表达水平(P值均<0.05)。扶正化瘀胶囊体外显著降低小鼠肝组织TGFβ1、PDFGB和TNFSF12 mRNA表达(P值均<0.01)。扶正化瘀胶囊减弱促炎BMDM细胞中TREM2荧光强度,并显著降低促炎BMDM中 ATF3的mRNA和蛋白表达(P值均<0.05)。 结论 扶正化瘀胶囊具有较好的改善小鼠代谢相关脂肪性肝病肝纤维化的作用,其作用机制可能与下调ATF3表达抑制SAM相关。 Abstract:Objective To investigate the molecular mechanism of Fuzheng Huayu Capsule in improving liver fibrosis in metabolic associated fatty liver disease by regulating scar-associated macrophages (SAMs). Methods A total of 24 C57 mice were randomly divided into control group (Con group), Model group, and Fuzheng Huayu Capsule group (FZHY group). Mice were given a high-fat diet and intraperitoneal injection of CCl4 for six weeks to establish a model of liver fibrosis in metabolic associated fatty liver disease. The drug was given by gavage for 5 consecutive weeks since week 2 of modeling. FZHY group was administered Fuzheng Huayu Capsule via oral gavage, while the Con and Model groups received an equal volume of saline solution via oral gavage. The serum levels of liver enzymes were measured, as well as the levels of triglyceride (TG) and hydroxyproline (Hyp) in the liver. HE staining and picrosirius red staining were used to observe liver tissue. Three liver tissue samples were collected from the Model group and the FZHY group, and transcriptome sequencing was performed to investigate the molecular mechanism of Fuzheng Huayu Capsule in improving liver fibrosis in metabolic associated fatty liver disease. Western blot and RT-qPCR were used to measure the protein and/or mRNA expression levels of SAM markers (CD9 and triggering receptor expressed on myeloid cells 2 [TREM2]), profibrogenic genes (transforming growth factor-β1 [TGFβ1], platelet-derived growth factor subunit beta [PDGFβ], and TNF superfamily member 12 [TNFSF12]), and the upstream regulator activating transcription factor 3 (ATF3) in liver tissue. The serum containing Fuzheng Huayu Capsule was used for the intervention of pro-inflammatory bone marrow-derived macrophages (BMDMs) induced by lipopolysaccharide and TGF-β1, and immunofluorescence assay, Western blot, and RT-qPCR were used to measure the expression levels of TREM2 and ATF3. The independent-samples t test was used for comparison of continuous data between two groups, and a one-way analysis of variance was used for comparison between multiple groups, while the least significant difference t-test was used for further comparison between two groups. Results Compared with the Model group, the FZHY group had significant reductions in the levels of liver enzymes, the levels of TG and Hyp in the liver, NAS score, and Sirius Red staining-positive area (all P<0.01). The RNA-seq analysis showed that differentially expressed genes were mainly enriched in chemokine signaling pathways, and Fuzheng Huayu Capsule significantly downregulated the expression of CCL2, CX3CL1, and CX3CR1(all P<0.01). Fuzheng Huayu Capsule significantly reduced the protein and mRNA expression levels of CD9, TREM2, and ATF3 in liver tissue (all P<0.05). In vitro, Fuzheng Huayu Capsule significantly reduced the mRNA expression levels of TGFβ1, PDGFβ, and TNFSF12 in liver tissue (all P<0.01). Fuzheng Huayu Capsule also attenuated TREM2 fluorescence intensity in pro-inflammatory BMDMs and significantly reduced the mRNA and protein expression levels of ATF3 (all P<0.05). Conclusion Fuzheng Huayu Capsule has a marked therapeutic effect on mice with liver fibrosis in metabolic associated fatty liver disease, possibly by downregulating the expression of ATF3 and inhibiting SAMs. -
表 1 引物序列
Table 1. Primer sequence
基因 上游引物(5′- 3′) 下游引物(5′- 3′) CD9 ACAAGTTCCACATCATTGGAG CAGGATCATGCTGAAGATCAT TREM2 CGGAATGGGAGCACAGTCATC CGGCTTGGAGGTTCTTCAGAG TGF-β1 ACCGCAACAACGCCATCTATGAG AGCCCTGTATTCCGTCTCCTTGG PDGFβ CTACCTACGCCCTGGTCAGC AGAATGTGCTATTCTCATGT TNFSF12 CGAGCTATTGCAACCCATTA TACAGGTAGTATTCCGCCAGC ATF3 CTCGGGGTGTCCATCACAAA GGCACTCCGTCTTCTCCTTC 表 2 各组小鼠血清ALT、AST水平及肝组织TG、Hyp含量比较
Table 2. Comparison of serum ALT and AST levels and liver tissue TG and Hyp contents in each group of mice
组别 ALT(U/L) AST(U/L) TG[mg/肝湿重(g)] Hyp[mg/肝湿重(g)] Con组 4.20±0.87 10.82±5.10 20.37±3.23 0.23±0.04 Model组 165.59±6.901) 84.70±6.011) 32.21±7.561) 0.46±0.031) FZHY组 115.60±5.082) 60.86±7.262) 21.64±7.142) 0.29±0.042) F值 275.10 390.80 9.12 97.65 P值 <0.01 <0.01 0.01 <0.01 注:与Con比较,1) P<0.01;与Model比较,2)P<0.01。
-
[1] ISRAELSEN M, FRANCQUE S, TSOCHATZIS EA, et al. Steatotic liver disease[J]. Lancet, 2024, 404( 10464): 1761- 1778. DOI: 10.1016/S0140-6736(24)01811-7. [2] HAGSTRÖM H, SHANG Y, HEGMAR H, et al. Natural history and progression of metabolic dysfunction-associated steatotic liver disease[J]. Lancet Gastroenterol Hepatol, 2024, 9( 10): 944- 956. DOI: 10.1016/S2468-1253(24)00193-6. [3] DO A, ZAHRAWI F, MEHAL WZ. Therapeutic landscape of metabolic dysfunction-associated steatohepatitis(MASH)[J]. Nat Rev Drug Discov, 2025, 24( 3): 171- 189. DOI: 10.1038/s41573-024-01084-2. [4] PARK MD, SILVIN A, GINHOUX F, et al. Macrophages in health and disease[J]. Cell, 2022, 185( 23): 4259- 4279. DOI: 10.1016/j.cell.2022.10.007. [5] TARU V, SZABO G, MEHAL W, et al. Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation[J]. J Hepatol, 2024, 81( 5): 895- 910. DOI: 10.1016/j.jhep.2024.06.016. [6] FABRE T, BARRON AMS, CHRISTENSEN SM, et al. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation[J]. Sci Immunol, 2023, 8( 82): eadd8945. DOI: 10.1126/sciimmunol.add8945. [7] CUI XY, SUN QH, ZHENG LH, et al. Role of triggering receptor expressed on myeloid cells 2 in acute and chronic liver diseases[J]. J Clin Hepatol, 2025, 41( 2): 383- 388. DOI: 10.12449/JCH250228.崔馨月, 孙全昊, 郑丽红, 等. 髓系细胞触发受体2(TREM2)在急慢性肝病中的作用[J]. 临床肝胆病杂志, 2025, 41( 2): 383- 388. DOI: 10.12449/JCH250228. [8] XIONG XL, KUANG H, ANSARI S, et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis[J]. Mol Cell, 2019, 75( 3): 644- 660.e5. DOI: 10.1016/j.molcel.2019.07.028. [9] RAMACHANDRAN P, DOBIE R, WILSON-KANAMORI JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level[J]. Nature, 2019, 575( 7783): 512- 518. DOI: 10.1038/s41586-019-1631-3. [10] XIN X, CAI BY, CHEN C, et al. Effect of fuzheng Huayu capsule on experimental non-alcoholic fatty liver fibrosis in mice[J]. Chin J Exp Tradit Med Formulae, 2021, 27( 6): 37- 45. DOI: 10.13422/j.cnki.syfjx.20210202.辛鑫, 蔡蓓玉, 陈成, 等. 扶正化瘀胶囊对非酒精性脂肪性肝纤维化小鼠的影响[J]. 中国实验方剂学杂志, 2021, 27( 6): 37- 45. DOI: 10.13422/j.cnki.syfjx.20210202. [11] BAILEY JD, SHAW A, MCNEILL E, et al. Isolation and culture of murine bone marrow-derived macrophages for nitric oxide and redox biology[J]. Nitric Oxide, 2020, 100: 17- 29. DOI: 10.1016/j.niox.2020.04.005. [12] WATANABE Y, TSUCHIYA A, SEINO S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice[J]. STEM CELLS Transl Med, 2019, 8( 3): 271- 284. DOI: 10.1002/sctm.18-0105. [13] ISHIYAMA S, HAYATSU M, TORIUMI T, et al. Assessing the combined impact of fatty liver-induced TGF-β1 and LPS-activated macrophages in fibrosis through a novel 3D serial section methodology[J]. Sci Rep, 2024, 14: 11404. DOI: 10.1038/s41598-024-60845-6. [14] GUAN X, LIU W, CHEN JM, et al. Research advances in the clinical and basic research on Fuzheng Huayu prescription in treatment of chronic liver diseases[J]. J Clin Hepatol, 2021, 37( 6): 1449- 1453. DOI: 10.3969/j.issn.1001-5256.2021.06.048.关茜, 刘伟, 陈佳美, 等. 扶正化瘀方治疗慢性肝病的临床与基础研究进展[J]. 临床肝胆病杂志, 2021, 37( 6): 1449- 1453. DOI: 10.3969/j.issn.1001-5256.2021.06.048. [15] RAO GC, PENG X, LI XQ, et al. Unmasking the Enigma of lipid metabolism in metabolic dysfunction-associated steatotic liver disease: From mechanism to the clinic[J]. Front Med, 2023, 10: 1294267. DOI: 10.3389/fmed.2023.1294267. [16] DENK H, ABUJA PM, ZATLOUKAL K. Animal models of NAFLD from the pathologist’s point of view[J]. Biochim Biophys Acta BBA Mol Basis Dis, 2019, 1865( 5): 929- 942. DOI: 10.1016/j.bbadis.2018.04.024. [17] TSUCHIDA T, LEE YA, FUJIWARA N, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer[J]. J Hepatol, 2018, 69( 2): 385- 395. DOI: 10.1016/j.jhep.2018.03.011. [18] TSOUKA S, KUMAR P, SEUBNOOCH P, et al. Transcriptomics-driven metabolic pathway analysis reveals similar alterations in lipid metabolism in mouse MASH model and human[J]. Commun Med, 2024, 4: 39. DOI: 10.1038/s43856-024-00465-3. [19] CHENG D, CHAI J, WANG HW, et al. Hepatic macrophages: Key players in the development and progression of liver fibrosis[J]. Liver Int, 2021, 41( 10): 2279- 2294. DOI: 10.1111/liv.14940. [20] REMMERIE A, MARTENS L, THONÉ T, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from kupffer cells in the fatty liver[J]. Immunity, 2020, 53( 3): 641- 657. e 14. DOI: 10.1016/j.immuni.2020.08.004. [21] GUILLIAMS M, BONNARDEL J, HAEST B, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches[J]. Cell, 2022, 185( 2): 379- 396. e 38. DOI: 10.1016/j.cell.2021.12.018. [22] LINK F, LI YJ, ZHAO JL, et al. ECM1 attenuates hepatic fibrosis by interfering with mediators of latent TGF-1 activation[J]. Gut, 2025, 74( 3): 424- 439. DOI: 10.1136/gutjnl-2024-333213. [23] LIU ZY, XIANG HY, XIANG DJ, et al. Revealing potential anti-fibrotic mechanism of Ganxianfang formula based on RNA sequence[J]. Chin Med, 2022, 17( 1): 23. DOI: 10.1186/s13020-022-00579-7. [24] GADIPUDI LL, RAMAVATH NN, PROVERA A, et al. Annexin A1 treatment prevents the evolution to fibrosis of experimental nonalcoholic steatohepatitis[J]. Clin Sci, 2022, 136( 9): 643- 656. DOI: 10.1042/cs20211122. [25] SEIDMAN JS, TROUTMAN TD, SAKAI M, et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis[J]. Immunity, 2020, 52( 6): 1057- 1074.e7. DOI: 10.1016/j.immuni.2020.04.001. [26] SHI ZM, ZHANG K, CHEN T, et al. Transcriptional factor ATF3 promotes liver fibrosis via activating hepatic stellate cells[J]. Cell Death Dis, 2020, 11: 1066. DOI: 10.1038/s41419-020-03271-6. [27] LI XM, LIN LF, LI YF, et al. ATF3-mediated transactivation of CXCL14 in HSCs during liver fibrosis[J]. Clin Transl Med, 2024, 14( 10): e70040. DOI: 10.1002/ctm2.70040. -



 PDF下载 ( 123601 KB)
PDF下载 ( 123601 KB)

 下载:
下载: