戊型肝炎病毒感染住院患者临床特征与医疗负担分析
DOI: 10.12449/JCH251213
Clinical features and medical burden of hospitalized patients with hepatitis E virus infection
-
摘要:
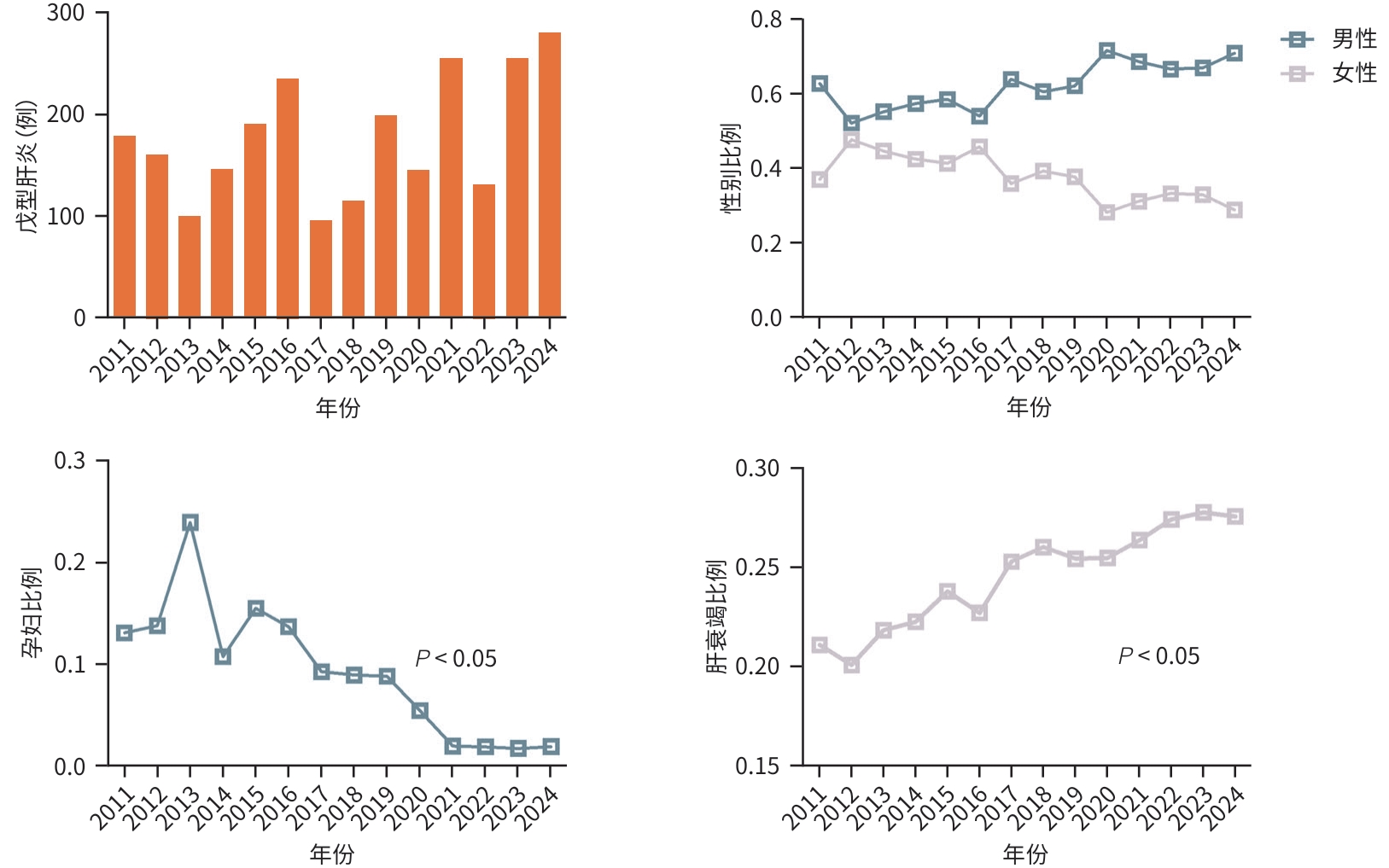
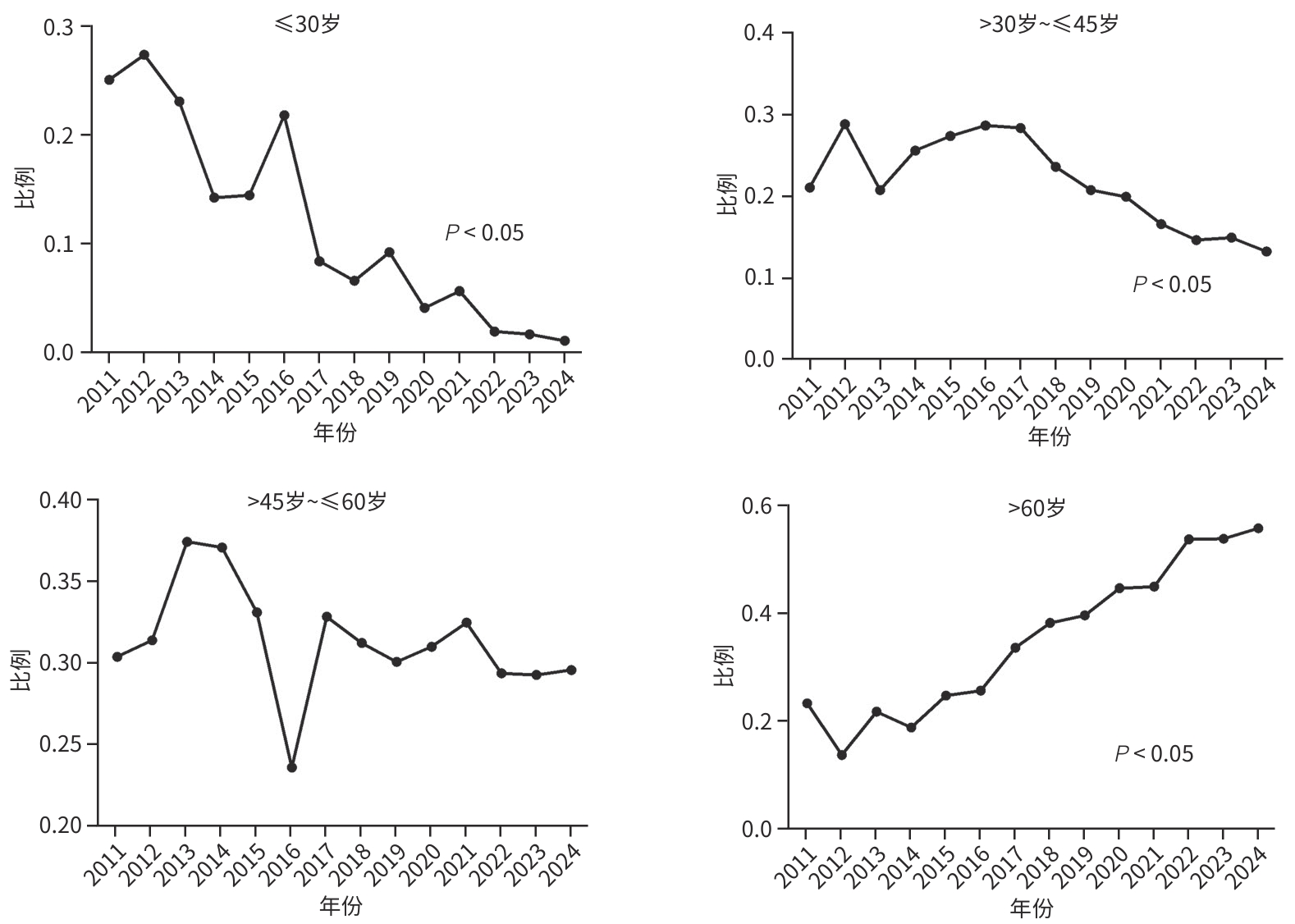
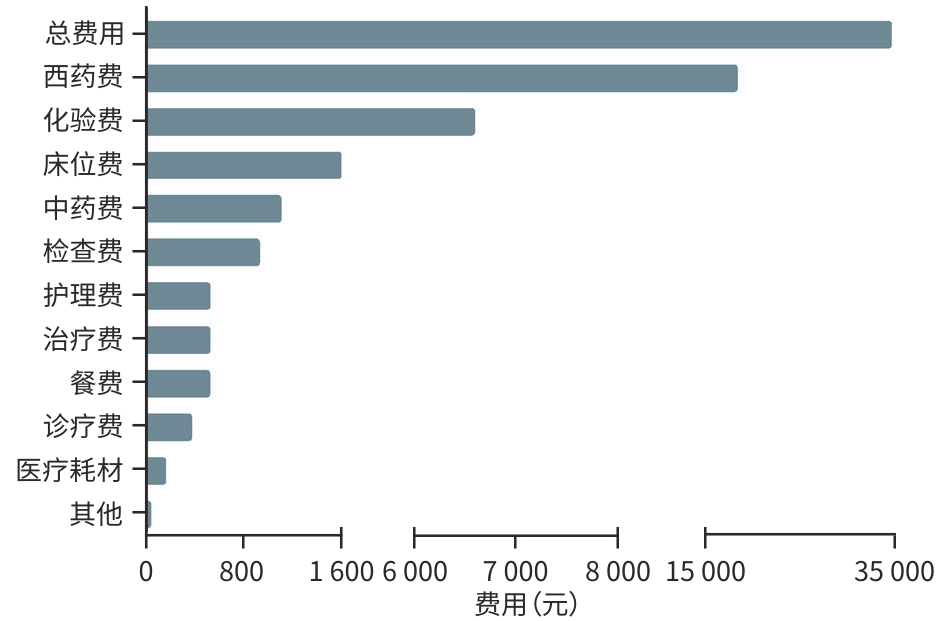
目的 分析戊型肝炎病毒(HEV)感染住院患者的人群分布特征、预后及医疗费用支出情况。 方法 回顾性收集2011年1月1日—2024年12月30日于上海市公共卫生临床中心住院的2 483例HEV感染者的病例资料,纳入人口统计学、临床症状、实验室检查、治疗情况及住院费用等信息进行统计学分析,计量资料多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Wilcoxon检验,计数资料组间比较采用χ²检验或Fisher确切概率法。 结果 近年来HEV在我国呈现散发流行,全国及上海地区历年病例数未见聚集性暴发。临床资料分析提示HEV感染住院患者以中老年男性为主(男性占比62.9%,60岁以上患者占比37.1%),60岁以上的老年感染者占比呈现逐年上升的趋势(P<0.05),组间比较显示孕产妇合并HEV感染率逐年下降(P<0.05),且HEV感染所致肝衰竭占总住院患者比例逐年升高(P<0.05);随访住院患者临床结局,显示肝衰竭患者不良预后发生率高(短期病死率达26.7%,χ2=465.8,P<0.001;肝移植率1.6%,χ2=20.4,P<0.001)。此外,HEV感染住院患者医疗费用支出大(平均总住院费用18 090元),合并肝衰竭者的医疗费用支出尤为突出(平均总住院费用34 383元,显著高于无症状感染组11 110元、急性无黄疸型肝炎组10 570元、急性黄疸型肝炎组15 139元、肝功能失代偿组19 314元;H=528.7,P<0.001),造成一定的卫生经济损失。 结论 HEV感染呈散发流行,历年HEV感染住院患者人口统计学特征呈现差异,肝衰竭发生率逐年增高且预后差,造成卫生经济负担重,值得临床重视。 Abstract:Objective To investigate the demographic distribution, prognosis and medical expenses of hospitalized patients with hepatitis E virus (HEV) infection. Methods A retrospective analysis was performed for the case data of 2 483 patients with HEV infection who were hospitalized in Shanghai Public Health Clinical Center from January 1, 2011 to December 30, 2024, and a statistical analysis was performed for demographic data, clinical symptoms, laboratory tests, treatment conditions, and hospital costs. The Kruskal-Wallis H test was used for comparison of continuous data between multiple groups, and the Wilcoxon test was used for further comparison between two groups; the chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups. Results Sporadic prevalence of HEV had been observed in China in recent years, without aggregated outbreaks in Shanghai and the whole country. The analysis of clinical data showed that middle-aged and elderly male patients were the main hospitalized population with HEV infection, with male patients accounting for 62.9% and patients aged >60 years accounting for 37.1%, and the poportion of patients aged >60 showed an increasing trend year by year (P<0.05). Comparison between groups showed a significant reduction in the rate of HEV infection year by year among pregnant women (P<0.05), while there was a gradual increase in the proportion of patients with liver failure caused by HEV infection among all hospitalized patients (P<0.05). Follow-up of the clinical outcomes of hospitalized patients showed a high incidence rate of adverse prognosis in patients with liver failure, with a short-term mortality rate of 26.7% (χ2=465.8, P<0.001) and a liver transplantation rate of 1.6% (χ2=20.4, P<0.001). In addition, hospitalized patients with HEV infection often had high medical expenses, with mean total hospital costs of 18 090 yuan, and those with liver failure had particularly high medical expenses, with mean total hospital costs of 34383 yuan, which was significantly higher than the medical expenses in the asymptomatic infection group (11 110 yuan), the acute non-jaundice hepatitis group (10 570 yuan), the acute jaundice hepatitis group (15 139 yuan), and the liver decompensation group (19 314 yuan) (H=528.7, P<0.001), causing a certain economic burden on health care. Conclusion HEV infection shows sporadic prevalence, and there are differences in the demographic features of hospitalized patients with HEV infection across the years. The incidence rate of liver failure has increased year by year, and such patients tend to have a poor prognosis, causing a heavy economic burden on health care, which should be taken seriously in clinical practice. -
Key words:
- Hepatitis E virus /
- Hepatitis E /
- Prognosis /
- Fees, Medical
-
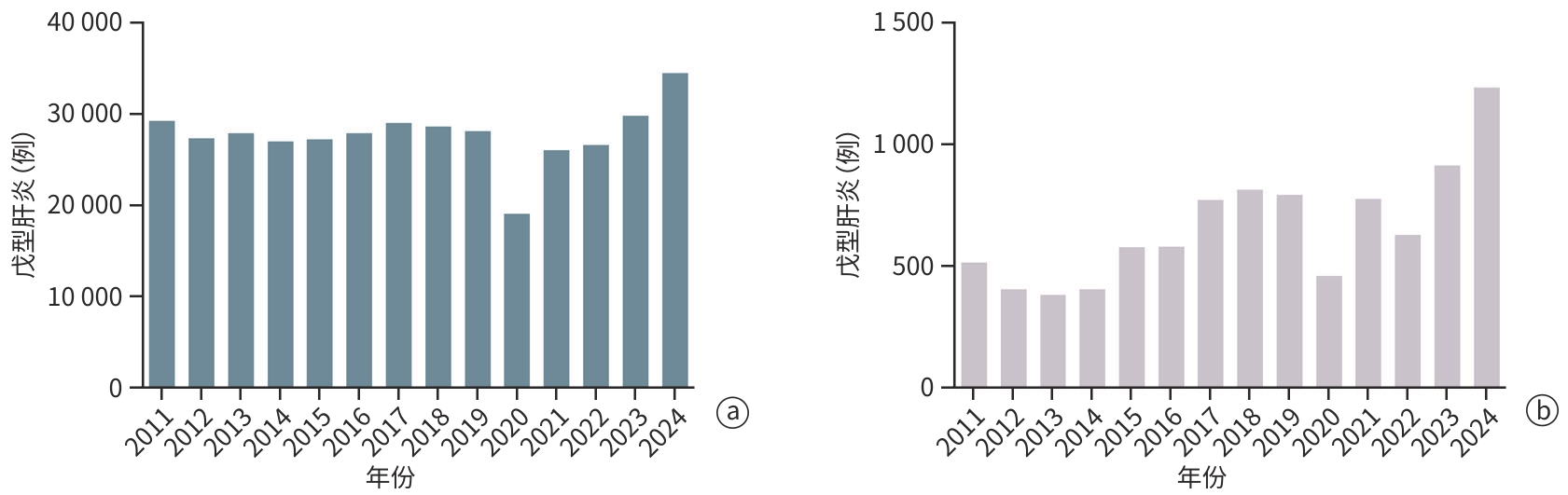
注: a,2011—2024年全国HEV感染数量分布;b,2011—2024年上海市HEV感染数量分布。数据来自中国疾病预防控制中心(网址:https://www.chinacdc.cn/)、中华人民共和国国家卫生和计划生育委员会(网址:https://www. nhc.gov.cn)与上海市卫生健康委数据监测(网址:https://wsjkw.sh.gov.cn/yqxx/)。
图 1 2011—2024年HEV感染者数量分布
Figure 1. Distribution of time and number of infections in patients with HEV infection
表 1 HEV感染住院患者临床特征
Table 1. Clinical characteristics hospitalized patients with HEV infection
指标 总患者
(n=2 483)无症状感染者
(n=418)急性无黄疸型
肝炎(n=295)急性黄疸型
肝炎(n=875)肝功能失代偿
(n=217)肝衰竭
(n=678)χ2值 P 值 男[例(%)] 1 562(62.9) 184(44.1) 219(74.2) 490(56.0) 137(63.1) 532(78.5) 223.5 <0.001 年龄[例(%)] ≤30岁 275(11.1) 95(22.7) 6(2.0) 132(15.1) 9(4.1) 33(4.9) 133.5 <0.001 >30岁~≤45岁 523(21.1) 114(27.3) 60(20.3) 191(21.8) 43(19.8) 115(17.0) 17.2 <0.01 >45岁~≤60岁 764(30.8) 85(20.3) 80(27.1) 297(33.9) 73(33.6) 229(33.8) 31.1 <0.001 >60岁 921(37.1) 124(29.7) 149(50.5) 255(29.1) 92(42.4) 301(44.4) 74.5 <0.001 临床转归[例(%)] 好转 2 283 (91.9) 418(100.0) 295(100.0) 875(100.0) 209(96.3) 486(71.7) 498.5 <0.001 肝移植 11(0.4) 0(0.0) 0(0.0) 0(0.0) 0(0.0) 11(1.6) 20.4 <0.001 死亡 189(7.6) 0(0.0) 0(0.0) 0(0.0) 8(3.7) 181(26.7) 465.8 <0.001 表 2 HEV感染住院患者医疗费用支出
Table 2. Medical expense expenditure of hospitalized patients infected with HEV
医疗支
出(元)总患者
(n=2 483)无症状感染者
(n=418)急性无黄疸型
肝炎(n=295)急性黄疸型肝炎
(n=875)肝功能失代偿
(n=217)肝衰竭
(n=678)H值 P 值 床位费 982
(527~1 825)600
(286~1 326)667
(360~1 131)890
(600~1 247)1)2)841
(540~1 419)1)2)1 580
(936~3 407)1)2)3)4)290.6 <0.001 餐费 315
(162~520)150
(78~261)225
(122~341)1)323
(216~450)1)2)308
(189~438)1)2)503
(310~796)1)2)3)4)421.2 <0.001 诊疗费 250
(110~561)148
(65~300)175
(85~350)1)261
(154~600)1)2)263
(116~725)1)2)355
(140~836)1)2)3)132.9 <0.001 治疗费 282
(135~947)423
(110~1 994)168
(85~395)1)197
(115~357)1)256
(156~519)1)2)3)507
(232~1 190)2)3)4)182.2 <0.001 护理费 343
(160~751)206
(110~410)260
(135~452)1)361
(199~771)1)2)359
(162~871)1)2)507
(198~1 188)1)2)3)4)138.0 <0.001 医疗
耗材费58
(0~397)245
(0~1935)0
(0~143)1)0
(0~115)1)2)58
(0~250)1)2)3)144
(0~513)1)2)3)4)204.5 <0.001 化验费 4 493
(3 094~6 775)2 773
(1 586~4 145)3 469
(2 220~5 249)1)4 293
(3 310~5 808)1)5 211
(3 609~
6 974)1)2)3)6 566
(4 594~9
679)1)2)3)4)530.2 <0.001 检查费 795
(430~1 449)739
(338~1 476)620
(345~1 098)683
(400~1 273)878
(497~1 541)3)910
(525~1 635)1)2)3)55.5 <0.001 西药费 7 202
(3 207~15 890)2 803
(780~5 367)3 366
(1 879~7 282)1)5 945
(3 630~9 990)1)2)8 534
(4 905~
14 871)1)2)3)18 050
(9 671~
35 036)1)2)3)4)702.2 <0.001 中药费 368
(0~1 971)158
(0~406)0
(0~223)1)638
(8~1 964)1)2)700
(0~2 759)1)2)1 087
(91~3 923)1)2)3)4)272.5 <0.001 其他 0
(0~31)8
(0~557)0
(0~17)1)0
(0~0)1)2)0
(0~0)1)3)0
(0~54)1)2)3)166.3 <0.001 总费用 18 090
(10 363~33 929)11 110
(6 095~18 873)10 570
(7 333~16 989)1)15 139
(10 533~22 617)1)2)19 314
(12 836~
31 081)1)2)3)34 383
(21 357~
61 724)1)2)3)4)528.7 <0.001 注:与无症状感染者比较,1)P<0.05;与急性无黄疸型肝炎比较,2)P<0.05;与急性黄疸型肝炎比较,3)P<0.05;与肝功能失代偿比较,4)P<0.05。
-
[1] SONGTANIN B, MOLEHIN AJ, BRITTAN K, et al. Hepatitis E virus infections: Epidemiology, genetic diversity, and clinical considerations[J]. Viruses, 2023, 15( 6): 1389. DOI: 10.3390/v15061389. [2] World Health Organization. Hepatitis E[EB/OL]. https://www.who.int/. https://www.who.int/ [3] ASLAN AT, BALABAN HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment[J]. World J Gastroenterol, 2020, 26( 37): 5543- 5560. DOI: 10.3748/wjg.v26.i37.5543. [4] DONG R, CHANG DC, LUO ZH, et al. The burden of HEV-related acute liver failure in Bangladesh, China and India: A systematic review and meta-analysis[J]. BMC Public Health, 2023, 23( 1): 2369. DOI: 10.1186/s12889-023-17302-2. [5] CHEN C, WANG ML, LI WX, et al. Hepatitis e virus infection increases the risk of obstetric complications and perinatal adverse outcomes in pregnant women with chronic hepatitis B virus infection[J]. Eur Rev Med Pharmacol Sci, 2024, 28( 5): 1904- 1912. DOI: 10.26355/eurrev_202403_35604. [6] ZHANG S, CHEN C, PENG J, et al. Investigation of underlying comorbidities as risk factors for symptomatic human hepatitis E virus infection[J]. Aliment Pharmacol Ther, 2017, 45( 5): 701- 713. DOI: 10.1111/apt.13938. [7] CHEN C, ZHANG SY, ZHANG DD, et al. Clinical features of acute hepatitis E super-infections on chronic hepatitis B[J]. World J Gastroenterol, 2016, 22( 47): 10388. DOI: 10.3748/wjg.v22.i47.10388. [8] LI Q, CHEN C, HUANG CL, et al. Noninvasive models for predicting poor prognosis of chronic HBV infection patients precipitating acute HEV infection[J]. Sci Rep, 2020, 10( 1): 2753. DOI: 10.1038/s41598-020-59670-4. [9] BUTI M, RUIZ-COBO JC, ESTEBAN R, et al. Hepatitis E as a trigger for acute-on-chronic liver failure[J]. Clin Mol Hepatol, 2025, 31: S196- S204. DOI: 10.3350/cmh.2024.0758. [10] DUAN BF, FENG Y. Current knowledge on the epidemiology and detection methods of hepatitis E virus in China[J]. Virol J, 2024, 21( 1): 307. DOI: 10.1186/s12985-024-02576-8. [11] HE ZW, LIU DY, LIU BL, et al. Prevalence of hepatitis E virus in swine in China: A systematic review with meta-analysis(2004-2023)[J]. Front Vet Sci, 2024, 11: 1472658. DOI: 10.3389/fvets.2024.1472658. [12] Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E[J]. Chin J Hepatol, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401.中华医学会肝病学分会. 戊型肝炎防治共识[J]. 中华肝脏病杂志, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401. [13] People’s Hospital of Peking University, Institute of Hepatology of Peking University, National Institute for the Control of Pharmaceutical and Biological Products, et al. Diagnostic Criteria for Hepatitis E[S]. 2008.北京大学人民医院, 北京大学肝病研究所, 中国药品生物制品检定所, 等. 戊型病毒性肝炎诊断标准[S]. 2008. [14] XU B. Clinical features and risk factors of acute hepatitis E with severe jaundice[J]. World J Gastroenterol, 2012, 18( 48): 7279. DOI: 10.3748/wjg.v18.i48.7279. [15] WU X, DING HG, LI WG, et al. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3760/cma.j.issn.1007-3418.2019.11.008.徐小元, 丁惠国, 李文刚, 等. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3760/cma.j.issn.1007-3418.2019.11.008. [16] The Subgroup of Severe Liver Disease and Artificial Liver in the Hepatology Branch of the Chinese Medical Association, and the Subgroup of Liver Failure and Artificial Liver in the Infectious Diseases Branch of the Chinese Medical Association. Guidelines for the diagnosis and treatment of liver failure(2024 Edition)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. [17] LIU TX, LI J, YIN X, et al. Establishment of enterically transmitted hepatitis virus animal models using lipid nanoparticle-based full-length viral genome RNA delivery system[J]. Gut, 2025, 74( 3): 467- 476. DOI: 10.1136/gutjnl-2024-332784. [18] WASUWANICH P, WEN TS, EGERMAN RS, et al. Epidemiology and outcomes of hepatitis E virus-associated hospitalisations in the United States with a focus on pregnancy: A nationwide population study, 1998-2020[J]. J Viral Hepat, 2024, 31( 11): 710- 719. DOI: 10.1111/jvh.13994. [19] KHONGVIWATSATHIEN S, THAWEERAT W, ATTHAKITMONGKOL T, et al. A comparison of clinical manifestations and outcomes between acute sporadic hepatitis a and hepatitis E infections in Thailand[J]. Viruses, 2023, 15( 9): 1888. DOI: 10.3390/v15091888. [20] LI XY, ZHOU Y, HUANG H, et al. Progress in disease burden researches[J]. Chin J Public Health, 2018, 34( 5): 777- 780. DOI: 10.11847/zgggws1118319.李茜瑶, 周莹, 黄辉, 等. 疾病负担研究进展[J]. 中国公共卫生, 2018, 34( 5): 777- 780. DOI: 10.11847/zgggws1118319. [21] LI YC. New progress in detection methods of viral hepatitis serum markers[J]. J Mol Diagn Ther, 2020, 12( 12): 1753- 1756.李轶春. 病毒性肝炎血清标志物检测方法新进展[J]. 分子诊断与治疗杂志, 2020, 12( 12): 1753- 1756. [22] Chinese Consortium for the Study of Hepatitis E(CCSHE), Chinese Physician Association for Infectious Disease, National Clinical Research Center for Infectious Diseases. Expert consensus on the process of in-hospital screening and management of viral hepatitis E in China(2023)[J]. J Clin Hepatol, 2023, 39( 4): 785- 794. DOI: 10.3969/j.issn.1001-5256.2023.04.008.中国戊型肝炎研究协助组(CCSHE), 中国医师协会感染科医师分会, 国家感染性疾病临床医学研究中心. 中国戊型病毒性肝炎院内筛查管理流程专家共识(2023年版)[J]. 临床肝胆病杂志, 2023, 39( 4): 785- 794. DOI: 10.3969/j.issn.1001-5256.2023.04.008. [23] GENG YS, SHI TF, WANG YC. Epidemiology of hepatitis E[M]// Hepatitis E Virus. Singapore: Springer Nature Singapore, 2023: 33- 48. DOI: 10.1007/978-981-99-1304-6_3. [24] HORVATITS T, SCHULZE ZUR WIESCH J, LÜTGEHETMANN M, et al. The clinical perspective on hepatitis E[J]. Viruses, 2019, 11( 7): 617. DOI: 10.3390/v11070617. [25] TOSONE G, SIMEONE D, SPERA AM, et al. Epidemiology and pathogenesis of fulminant viral hepatitis in pregnant women[J]. Minerva Obstet Gynecol, 2018, 70( 4): 480- 486. DOI: 10.23736/s0026-4784.17.04107-7. [26] YANG S. Prevention strategies for hepatitis E[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2023, 17( 4): 287- 288. DOI: 10.3877/cma.j.issn.1674-1358.2023.04.011.杨松. 戊型病毒性肝炎的预防策略[J/CD]. 中华实验和临床感染病杂志(电子版), 2023, 17( 4): 287- 288. DOI: 10.3877/cma.j.issn.1674-1358.2023.04.011. [27] WANG YY, HUANG J, LAN Y, et al. Investigation and analysis on the awareness rate of hepatitis E prevention knowledge and vaccination willingness of employees in Nanning[J]. Appl Prev Med, 2025, 31( 1): 91- 95.王芸芸, 黄菊, 蓝莹, 等. 南宁市从业人员戊型肝炎防治知识知晓率及疫苗接种意愿调查分析[J]. 应用预防医学, 2025, 31( 1): 91- 95. [28] CHEN Q. Analysis on detection of Hepatitis A and E and influencing factors of"knowledge, attitude and behavior" in some practitioners in a district of Hangzhou, 2015-2016[J]. Chin J Public Health Manag, 2018, 34( 2): 271- 273. DOI: 10.19568/j.cnki.23-1318.2018.02.037.陈琼. 杭州市某区2015—2016年部分从业者甲(戊)型肝炎检测现况与“知信行”影响因素分析[J]. 中国公共卫生管理, 2018, 34( 2): 271- 273. DOI: 10.19568/j.cnki.23-1318.2018.02.037. [29] ZHU YP, ZHU CW. Advances in the prevention and treatment of the high-risk population for hepatitis E[J]. J Clin Hepatol, 2023, 39( 11): 2524- 2529. DOI: 10.3969/j.issn.1001-5256.2023.11.002.朱月萍, 朱传武. 戊型肝炎高风险人群的防治进展[J]. 临床肝胆病杂志, 2023, 39( 11): 2524- 2529. DOI: 10.3969/j.issn.1001-5256.2023.11.002. [30] CHIBBER RM, USMANI MA, AL-SIBAI MH. Should HEV infected mothers breast feed?[J]. Arch Gynecol Obstet, 2004, 270( 1): 15- 20. DOI: 10.1007/s00404-002-0466-5. [31] ZHONG GH, ZHUANG CL, HU XW, et al. Safety of hepatitis E vaccination for pregnancy: A post-hoc analysis of a randomized, double-blind, controlled phase 3 clinical trial[J]. Emerg Microbes Infect, 2023, 12( 1): 2185456. DOI: 10.1080/22221751.2023.2185456. -



 PDF下载 ( 1134 KB)
PDF下载 ( 1134 KB)

 下载:
下载: