HBeAg阴性慢性乙型肝炎初治不完全应答患者联合或序贯抗病毒疗效比较分析
DOI: 10.12449/JCH251211
Efficacy of combined versus sequential antiviral therapy in HBeAg-negative chronic hepatitis B patients achieving incomplete virological response during initial antiviral therapy
-
摘要:
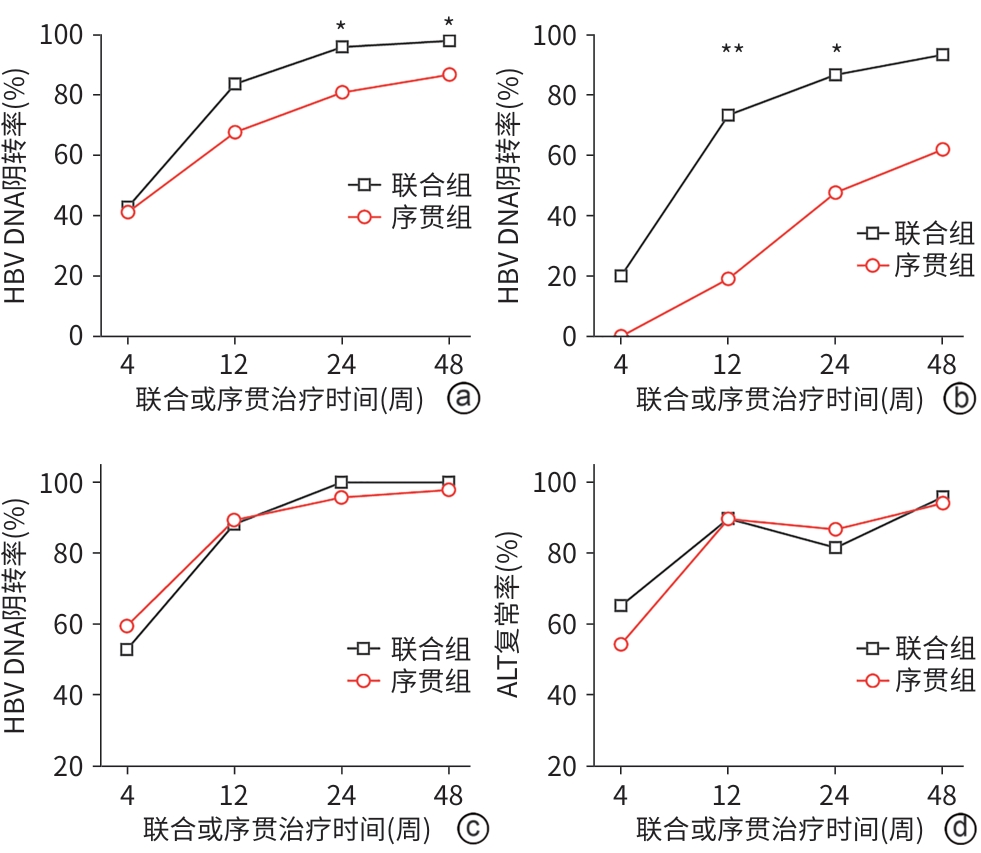
目的 探讨HBeAg阴性慢性乙型肝炎(CHB)患者在初次抗病毒治疗发生不完全应答后,采用联合或序贯方案的疗效及安全性差异,为临床实践提供证据支持。 方法 纳入2020年1月—2023年12月在中国人民解放军联勤保障部队第九八〇医院肝病科门诊就诊的117例符合不完全应答标准的HBeAg阴性CHB患者(抗病毒治疗1年HBV DNA≥20 IU/mL),根据临床实际,将患者分为联合组(原抗病毒药物联合第二种药物)和序贯组(停用原药换用新药)。主要疗效指标为联合或序贯治疗4、12、24、48周时的HBV DNA阴转率,次要疗效指标为各随访时间点患者ALT复常率及肾功能、心肌酶和血脂水平。符合正态分布的计量资料2组间比较采用成组t检验;计数资料2组间比较采用χ2检验和Fisher精确概率检验。 结果 117例CHB患者中,联合组49例,序贯组68例。联合或序贯治疗24、48周,联合组HBV DNA阴转率显著高于序贯组(24周:95.92% vs 80.88%,χ2=5.761,P=0.016;48周:97.96% vs 86.76%,χ2=4.566,P=0.044),组间比较差异均有统计学意义。与序贯组相比,联合治疗对高病毒载量(HBV DNA≥104 IU/mL)患者的病毒抑制水平更显著(12周:73.33% vs 19.05%,χ2=10.609,P=0.002;24周:86.67% vs 47.62%,χ2=5.783,P=0.033)。在低病毒载量患者中,联合或序贯方案组间疗效无明显差异(P值均>0.05)。联合组4、12、24、48周随访点ALT复常率分别为65.31%、89.80%、81.63%和95.92%,序贯组分别为54.41%、89.71%、86.76%和94.12%,2组间比较,差异均无统计学意义(P值均>0.05)。联合和序贯治疗48周内,两组均未出现肾功能、心肌酶、血脂等相关指标异常。 结论 对于HBeAg阴性CHB初治不完全应答患者,联合治疗在高病毒载量亚组中显著优于序贯治疗,能更快实现病毒学抑制;而低病毒载量患者中两者疗效趋同。联合和序贯治疗总体安全性良好。 Abstract:Objective To investigate the efficacy and safety of combined versus sequential antiviral regimens in HBeAg-negative chronic hepatitis B (CHB) patients achieving incomplete virological response during initial antiviral therapy, and to provide evidence for clinical practice. Methods A total of 117 HBeAg-negative CHB patients who attended The 980th Hospital of PLA Joint Logistics Support Force from January 2020 to December 2023 and met the criteria for incomplete virological response were enrolled, and according to the clinical treatment regimen, they were divided into combination group (the original antiviral agent combined with another type of drug) and sequential group (switching to a new drug). The primary outcome measure was HBV DNA clearance rate at weeks 4, 12, 24, and 48 of combined or sequential therapy, and the secondary outcome measures were alanine aminotransferase (ALT) normalization rate, renal function, myocardial enzymes, and blood lipid profiles. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the chi-square test and the Fisher’s exact test were used for comparison of categorical data between two groups. Results Among the 117 CHB patients, there were 49 patients in the combination group and 68 patients in the sequential group. At weeks 24 and 48 of combined or sequential therapy, the combination group had a significantly higher HBV DNA clearance rate than the sequential group (week 24: 95.92% vs 80.88%, χ2=5.761, P=0.016; week 48: 97.96% vs 86.76%, χ2=4.566, P=0.044). Compared with the sequential group, the combination group showed a significantly higher level of viral inhibition in patients with a high viral load (HBV DNA ≥104 IU/mL) (week 12: 73.33% vs 19.05%, χ2=10.609, P=0.002; week 24: 86.67% vs 47.62%, χ2=5.783, P=0.033). There was no significant difference in treatment outcome between the two groups among the patients with a low viral load (P>0.05). The combination group had an ALT normalization rate of 65.31%, 89.80%, 81.63%, and 95.92%, respectively, at 4, 12, 24, and 48 weeks, while the sequential group had an ALT normalization rate of 54.41%, 89.71%, 86.76%, and 94.12%, respectively; there were no significant differences between the two groups (all P>0.05). There were no abnormalities in renal function, myocardial enzymes, and blood lipids in either group within 48 weeks of combined or sequential therapy. Conclusion In HBeAg-negative CHB patients with incomplete virological response, combination therapy has significantly better efficacy than sequential therapy in the high viral load subgroup and can achieve virological suppression more rapidly, and the two regimens have similar efficacy in patients with a low viral load. Both combined and sequential therapies have a favorable safety profile. -
Key words:
- Hepatitis B, Chronic /
- Hepatitis B e Antigens /
- Antiviral Agents /
- Treatment Outcome
-
表 1 117例HBeAg阴性CHB患者基线特征
Table 1. Demographic and clinical characteristics of 117 HBeAg-negative CHB patients
指标 联合组(n=49) 序贯组(n=68) 统计值 P值 性别(男/女,例) 32/17 47/21 χ2=0.189 0.664 年龄(岁) 48.06±10.81 45.97±9.95 t=1.082 0.282 初治药物(ETV/TDF/TAF,例) 20/12/17 28/17/23 χ2=0.010 0.995 HBV DNA(log10 IU/mL) 3.15±1.32 3.29±1.29 t=-0.595 0.553 ALT(U/L) 48.41±22.59 43.94±20.50 t=1.114 0.268 血清白蛋白(g/L) 41.20±1.67 40.95±1.79 t=0.744 0.458 总胆红素(μmol/L) 14.42±4.45 15.23±4.59 t=-0.948 0.345 血小板计数(×109/L) 181.67±39.23 183.71±39.52 t=-0.275 0.784 血肌酐(μmol/L) 54.98±13.18 58.00±12.40 t=-1.266 0.208 肌酸磷酸肌酶(U/L) 146.12±67.89 166.13±69.90 t=-1.546 0.125 总胆固醇(mmol/L) 4.47±1.17 4.57±1.61 t=-0.370 0.712 甘油三酯(mmol/L) 1.29±0.54 1.19±0.56 t=0.987 0.326 表 2 联合或序贯治疗患者HBV DNA应答情况
Table 2. Virological responses of patients receiving combination or sequential therapy
项目 随访时间(周) 联合组
HBV DNA阴转率
序贯组
HBV DNA阴转率
χ2值 P值 总计 4 42.86%(21/49) 41.18%(28/68) 0.033 0.856 12 83.67%(41/49) 67.65%(46/68) 3.836 0.050 24 95.92%(47/49) 80.88%(55/68) 5.761 0.016 48 97.96%(48/49) 86.76%(59/68) 4.566 0.044 高HBV DNA亚组 4 20.00%(3/15) 0.00%(0/21) 4.582 0.064 12 73.33%(11/15) 19.05%(4/21) 10.609 0.002 24 86.67%(13/15) 47.62%(10/21) 5.783 0.033 48 93.33%(14/15) 61.90%(13/21) 4.610 0.051 低HBV DNA亚组 4 52.94%(18/34) 59.57%(28/47) 0.354 0.552 12 88.24%(30/34) 89.36%(42/47) 0.025 >0.05 24 100.00%(34/34) 95.74%(45/47) 1.483 0.507 48 100.00%(34/34) 97.87%(46/47) 0.732 >0.05 ETV亚组 4 45.00%(9/20) 53.57%(15/28) 0.343 0.558 12 90.00%(18/20) 78.57%(22/28) 1.097 0.440 24 95.00%(19/20) 89.29%(25/28) 0.499 0.631 48 95.00%(19/20) 89.29%(25/28) 0.499 0.631 TDF亚组 4 33.33%(4/12) 41.18%(7/17) 0.184 0.717 12 83.33%(10/12) 52.94%(9/17) 2.876 0.126 24 100.00%(12/12) 64.71%(11/17) 5.340 0.028 48 100.00%(12/12) 70.59%(12/17) 4.265 0.059 TAF亚组 4 47.06%(8/17) 26.09%(6/23) 1.890 0.169 12 76.47%(13/17) 65.22%(15/23) 0.589 0.443 24 94.12%(16/17) 82.61%(19/23) 1.184 0.373 48 100.00%(17/17) 95.65%(22/23) 0.758 >0.05 表 3 联合或续贯治疗患者48周生化学指标比较
Table 3. Comparison of 48-week biochemical safety profiles between patients receiving combination or sequential therapy
指标 联合组
(n=49)序贯组
(n=68)t值 P值 血肌酐(μmol/L) 56.69±14.26 60.75±15.29 -1.456 0.148 肌酸磷酸肌酶(U/L) 165.10±74.16 153.85±66.87 0.858 0.393 总胆固醇(mmol/L) 3.85±1.33 4.10±1.10 -1.147 0.254 甘油三酯(mmol/L) 1.36±0.43 1.46±0.44 -1.257 0.211 -
[1] van BÖMMEL F, STEIN K, HEYNE R, et al. A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B[J]. J Hepatol, 2023, 78( 5): 926- 936. DOI: 10.1016/j.jhep.2022.12.018. [2] MUTIMER D, ELSHARKAWY A, HATHORN E, et al. Rate and determinants of antiviral treatment initiation for patients with HBeAg-negative chronic hepatitis B[J]. J Viral Hepat, 2023, 30( 8): 694- 699. DOI: 10.1111/jvh.13841. [3] ZHAO XY, LI M, WANG H, et al. Impact of national centralized drug procurement policy on antiviral utilization and expenditure for hepatitis B in China[J]. J Clin Transl Hepatol, 2022, 10( 3): 420- 428. DOI: 10.14218/JCTH.2022.00167. [4] ZHANG Y, YANG S. Interpretation of 2024 World Health Organization guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2024, 18( 3): 129- 134. DOI: 10.3877/cma.j.issn.1674-1358.2024.03.001.张雨, 杨松. 世界卫生组织《慢性乙型肝炎预防、诊断、关怀及治疗指南(2024年版)》解读[J/CD]. 中华实验和临床感染病杂志(电子版), 2024, 18( 3): 129- 134. DOI: 10.3877/cma.j.issn.1674-1358.2024.03.001. [5] JIN MH, JIANG SW, HU AR, et al. Research progress on differential improvement and mechanism of nucleoside analogues or nucleotide analogues in HBV-related hepato-cellular carcinoma[J]. Chin J Clin Pharmacol Ther, 2025, 30( 6): 835- 848. DOI: 10.12092/j.issn.1009-2501.2025.06.014.金梦涵, 蒋素文, 胡爱荣, 等. 核苷与核苷酸类抗病毒药物对乙型肝炎相关肝细胞癌的差异性改善及其机制[J]. 中国临床药理学与治疗学, 2025, 30( 6): 835- 848. DOI: 10.12092/j.issn.1009-2501.2025.06.014. [6] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [7] LU FM, FENG B, ZHENG SJ, et al. Current status of the research on low-level viremia in chronic hepatitis B patients receiving nucleos(t)ide analogues[J]. J Clin Hepatol, 2021, 37( 6): 1268- 1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007.鲁凤民, 封波, 郑素军, 等. 核苷(酸)类似物经治的慢性乙型肝炎患者低病毒血症的研究现状[J]. 临床肝胆病杂志, 2021, 37( 6): 1268- 1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007. [8] HE MW, CUI L, CHEN DD, et al. Efficacy and safety of switching from entecavir to tenofovir alafenamide in chronic hepatitis B patients with low-level viremia: A real-world 48-week extension study[J]. Antimicrob Agents Chemother, 2025, 69( 3): e01827-24. DOI: 10.1128/aac.01827-24. [9] LI ZB, CHEN DD, JIA YF, et al. Risk factors related to low-level viraemia in chronic hepatitis B patients receiving entecavir treatment[J]. Front Cell Infect Microbiol, 2024, 14: 1413589. DOI: 10.3389/fcimb.2024.1413589. [10] WONG GL, LEMOINE M. The 2024 updated WHO guidelines for the prevention and management of chronic hepatitis B: Main changes and potential implications for the next major liver society clinical practice guidelines[J]. J Hepatol, 2025, 82( 5): 918- 925. DOI: 10.1016/j.jhep.2024.12.004. [11] LUMLEY SF, MOKAYA J, MAPONGA TG, et al. Hepatitis B virus resistance to nucleos(t)ide analogue therapy: WHO consultation on questions, challenges, and a roadmap for the field[J]. Lancet Microbe, 2025, 6( 8): 101076. DOI: 10.1016/j.lanmic.2025.101076. [12] CHOI J, CHOI WM, LIM YS. Are the new nucleos(t)ide analogs better than the old nucleos(t)ide analogs?[J]. Clin Liver Dis, 2023, 27( 4): 809- 818. DOI: 10.1016/j.cld.2023.05.005. [13] HUANG SC, KAO JH. Combining therapeutic agents to target the immune systems of hepatitis B patients: What do we need to consider?[J]. Expert Rev Gastroenterol Hepatol, 2025, 19( 4): 371- 375. DOI: 10.1080/17474124.2025.2477256. [14] EMERY JS, FELD JJ. Treatment of hepatitis B virus with combination therapy now and in the future[J]. Best Pract Res Clin Gastroenterol, 2017, 31( 3): 347- 355. DOI: 10.1016/j.bpg.2017.04.007. [15] ALI SH, SHAH MH, ROY S, et al. Efficacy and safety of tenofovir plus entecavir combination therapy versus tenofovir monotherapy in chronic hepatitis B virus patients with resistance or partial response to entecavir: A systematic review and meta-analysis[J]. J Clin Exp Hepatol, 2025, 15( 4): 102541. DOI: 10.1016/j.jceh.2025.102541. [16] WANG YH, LIAO J, ZHANG DM, et al. Tenofovir monotherapy versus tenofovir plus entecavir combination therapy in HBeAg-positive chronic hepatitis patients with partial virological response to entecavir[J]. J Med Virol, 2020, 92( 3): 302- 308. DOI: 10.1002/jmv.25608. [17] WOO HY, PARK JY, BAE SH, et al. Entecavir+tenofovir vs. lamivudine/telbivudine+adefovir in chronic hepatitis B patients with prior suboptimal response[J]. Clin Mol Hepatol, 2020, 26( 3): 352- 363. DOI: 10.3350/cmh.2019.0044n. [18] LIU LP. Analysis of efficacy and factors influencing sequential combination therapy with tenofovir alafenamide fumarate after treatment with entecavir in chronic hepatitis B patients with low-level viremia[D]. Nanchang: Medical School of Nanchang University, 2024.刘丽萍. 恩替卡韦经治后低病毒血症的慢性乙型肝炎患者序贯联合富马酸丙酚替诺福韦治疗的疗效及影响因素分析[D]. 南昌: 南昌大学医学部, 2024. [19] BAZINET M, ANDERSON M, PÂNTEA V, et al. Analysis of HBsAg immuno complexes and cccDNA activity during and persisting after NAP-based therapy[J]. Hepatol Commun, 2021, 5( 11): 1873- 1887. DOI: 10.1002/hep4.1767. [20] LIU T, WANG H, ZHAO Y, et al. Drug development for chronic hepatitis B functional cure: Recent progress[J]. World J Hepatol, 2025, 17( 4): 105797. DOI: 10.4254/wjh.v17.i4.105797. [21] LI S, SHI LC, HUANG C, et al. Impact of hepatitis B surface antigen quantification on achieving a functional cure in patients with chronic hepatitis B: A systematic review and meta-analysis[J]. Ann Hepatol, 2025, 30( 2): 101921. DOI: 10.1016/j.aohep.2025.101921. -



 PDF下载 ( 932 KB)
PDF下载 ( 932 KB)

 下载:
下载:


