胆道恶性肿瘤的治疗现状与未来展望
DOI: 10.12449/JCH251201
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:马文聪、章程负责查阅文献,撰写论文;王敬晗负责起草文章大纲,审阅修改文章;姜小清指导文章撰写,并最后定稿。
-
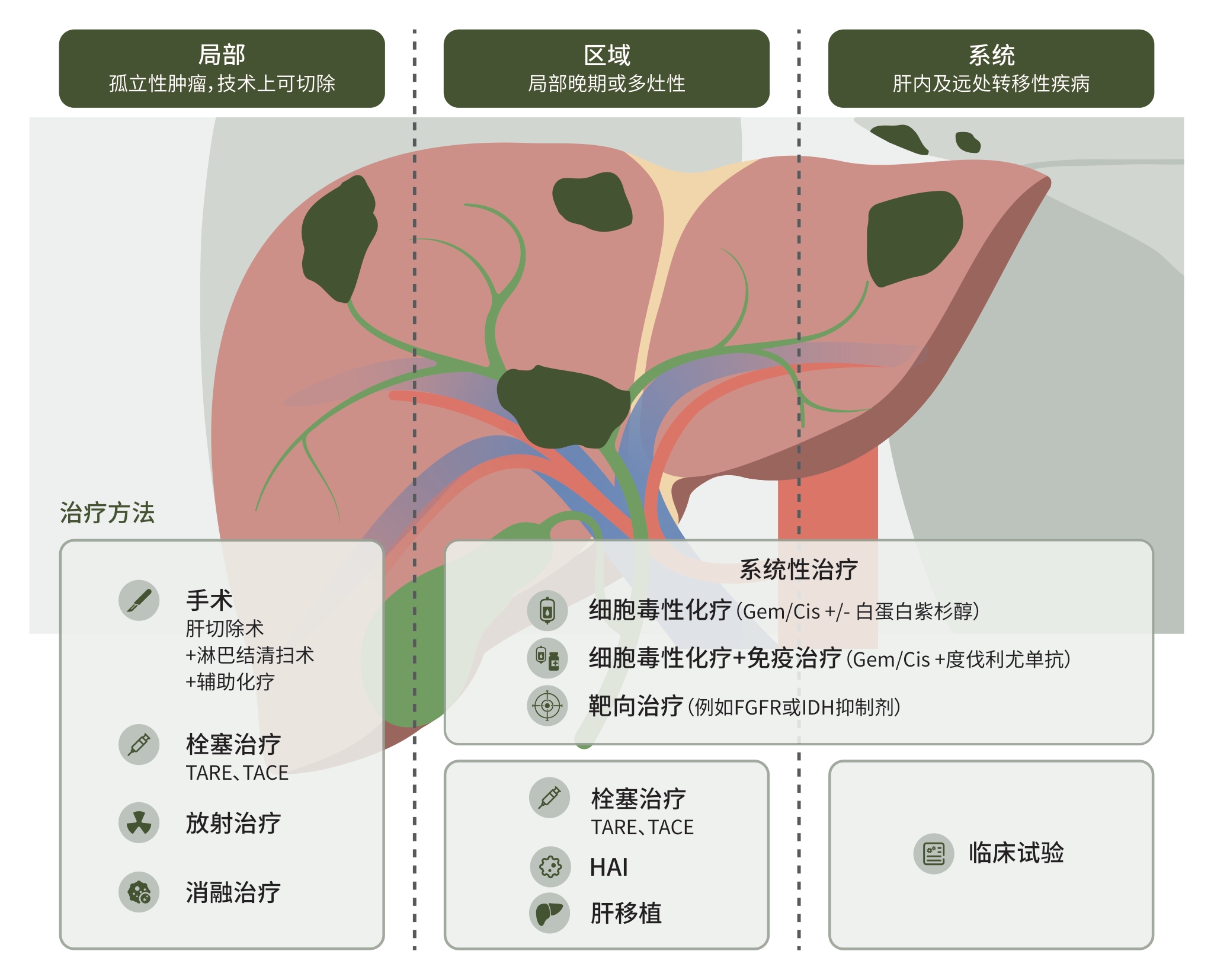
摘要: 胆道恶性肿瘤是一类具有高度侵袭性和异质性的肿瘤,发病率呈逐年上升态势。近年来,随着靶向治疗和免疫治疗的突破性进展,以及基因检测技术的广泛应用,胆道恶性肿瘤的治疗已从传统的手术、局部治疗,过渡到多种治疗方式联合治疗的阶段,为不同分期患者提供更加合理有效的治疗方案。本文旨在通过回顾目前胆道恶性肿瘤治疗的循证学证据,分析治疗的现状,并探讨胆道恶性肿瘤治疗未来发展方向。Abstract: Biliary tract cancer (BTC) is a type of tumor with high invasiveness and heterogeneity, and its incidence rate is increasing year by year. In recent years, with the breakthroughs in targeted therapy and immunotherapy, as well as the wide application of genetic testing techniques, the treatment of BTC has evolved from traditional surgery and local treatment to a stage of the combination of multiple treatment methods, providing more reasonable and effective treatment regimens for patients at different stages. This article reviews the current evidence-based medical data in the treatment of BTC, analyzes the current status of treatment, and discusses the future development directions of BTC treatment.
-
Key words:
- Biliary Tract Neoplasms /
- Molecular Targeted Therapy /
- Immunotherapy
-
[1] VALLE JW, KELLEY RK, NERVI B, et al. Biliary tract cancer[J]. Lancet, 2021, 397( 10272): 428- 444. DOI: 10.1016/s0140-6736(21)00153-7. [2] HENNEDIGE TP, NEO WT, VENKATESH SK. Imaging of malignancies of the biliary tract- an update[J]. Cancer Imag, 2014, 14( 1): 14. DOI: 10.1186/1470-7330-14-14. [3] BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. [4] VOGEL A, BRIDGEWATER J, EDELINE J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2023, 34( 2): 127- 140. DOI: 10.1016/j.annonc.2022.10.506. [5] FLORIO AA, FERLAY J, ZNAOR A, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012[J]. Cancer, 2020, 126( 11): 2666- 2678. DOI: 10.1002/cncr.32803. [6] KHAN SA, TOLEDANO MB, TAYLOR-ROBINSON SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma[J]. Hpb, 2008, 10( 2): 77- 82. DOI: 10.1080/13651820801992641. [7] RANDI G, MALVEZZI M, LEVI F, et al. Epidemiology of biliary tract cancers: An update[J]. Ann Oncol, 2009, 20( 1): 146- 159. DOI: 10.1093/annonc/mdn533. [8] KHAN SA, TAVOLARI S, BRANDI G. Cholangiocarcinoma: Epidemiology and risk factors[J]. Liver Int, 2019, 39( S1): 19- 31. DOI: 10.1111/liv.14095. [9] LEE SS, KIM MH, LEE SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis[J]. Cancer, 2004, 100( 4): 783- 793. DOI: 10.1002/cncr.20031. [10] LAMARCA A, EDELINE J, MCNAMARA MG, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers[J]. Cancer Treat Rev, 2020, 84: 101936. DOI: 10.1016/j.ctrv.2019.101936. [11] JAVLE M, LEE S, AZAD NS, et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017[J]. Oncologist, 2022, 27( 10): 874- 883. DOI: 10.1093/oncolo/oyac150. [12] IZQUIERDO-SANCHEZ L, LAMARCA A, LA CASTA A, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry[J]. J Hepatol, 2022, 76( 5): 1109- 1121. DOI: 10.1016/j.jhep.2021.12.010. [13] LI B, LI ZS, QIU ZQ, et al. Surgical treatment of hilar cholangiocarcinoma: Retrospective analysis[J]. BJS Open, 2023, 7( 3): zrad024. DOI: 10.1093/bjsopen/zrad024. [14] LIU Q, JIANG N, TIAN EY, et al. Short-term outcomes of robotic versus open pancreaticoduodenectomy in elderly patients: A multicenter retrospective cohort study[J]. Int J Surg, 2022, 104: 106819. DOI: 10.1016/j.ijsu.2022.106819. [15] KABIR T, TAN HL, SYN NL, et al. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies[J]. Surgery, 2022, 171( 2): 476- 489. DOI: 10.1016/j.surg.2021.07.020. [16] ZUREIKAT AH, POSTLEWAIT LM, LIU Y, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy[J]. Ann Surg, 2016, 264( 4): 640- 649. DOI: 10.1097/sla.0000000000001869. [17] HEIMBACH J, GORES G, HADDOCK M, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma[J]. Semin Liver Dis, 2004, 24( 2): 201- 207. DOI: 10.1055/s-2004-828896. [18] VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/nejmoa0908721. [19] KIM ST, KANG JH, LEE J, et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial[J]. Ann Oncol, 2019, 30( 5): 788- 795. DOI: 10.1093/annonc/mdz058. [20] LAMARCA A, PALMER DH, WASAN HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer(ABC-06): A phase 3, open-label, randomised, controlled trial[J]. Lancet Oncol, 2021, 22( 5): 690- 701. DOI: 10.1016/s1470-2045(21)00027-9. [21] HYUNG J, KIM I, KIM KP, et al. Treatment with liposomal irinotecan plus fluorouracil and leucovorin for patients with previously treated metastatic biliary tract cancer: The phase 2b NIFTY randomized clinical trial[J]. JAMA Oncol, 2023, 9( 5): 692. DOI: 10.1001/jamaoncol.2023.0016. [22] YANG JF, WANG J, ZHOU HB, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: A randomized trial[J]. Endoscopy, 2018, 50( 8): 751- 760. DOI: 10.1055/s-0043-124870. [23] GAO DJ, YANG JF, MA SR, et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: A multicenter randomized controlled trial[J]. Gastrointest Endosc, 2021, 94( 1): 91- 100.e2. DOI: 10.1016/j.gie.2020.12.016. [24] ORTNER MEJ, LIEBETRUTH J, SCHREIBER S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma[J]. Gastroenterology, 1998, 114( 3): 536- 542. DOI: 10.1016/s0016-5085(98)70537-2. [25] ZOEPF T, JAKOBS R, ARNOLD JC, et al. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy[J]. Am J Gastroenterology, 2005, 100( 11): 2426- 2430. DOI: 10.1111/j.1572-0241.2005.00318.x. [26] NANASHIMA A, YAMAGUCHI H, SHIBASAKI S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: A preliminary study[J]. J Gastroenterol, 2004, 39( 11): 1095- 1101. DOI: 10.1007/s00535-004-1449-z. [27] LI ZY, JIANG XF, XIAO H, et al. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma[J]. Surg Endosc, 2021, 35( 10): 5655- 5664. DOI: 10.1007/s00464-020-08095-1. [28] WIEDMANN M, CACA K, BERR F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: A Phase II pilot study[J]. Cancer, 2003, 97( 11): 2783- 2790. DOI: 10.1002/cncr.11401. [29] GUPTA P, MARALAKUNTE M, SAGAR S, et al. Efficacy and safety of irreversible electroporation for malignant liver tumors: A systematic review and meta-analysis[J]. Eur Radiol, 2021, 31( 9): 6511- 6521. DOI: 10.1007/s00330-021-07742-y. [30] FRANZESE C, BONU ML, COMITO T, et al. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: Single-institution analysis of outcome and toxicity[J]. J Cancer Res Clin Oncol, 2020, 146( 9): 2289- 2297. DOI: 10.1007/s00432-020-03285-9. [31] MENG SY, DING GC, SHI CL, et al. Observation of the efficacy of stereotactic radiotherapy combined with chemotherapy for locally advanced perihilar cholangiocarcinoma[J]. Acad J Chinese PLA Postgrad Med Sch, 2019, 40( 11): 1014- 1017, 1033. DOI: 10.3969/j.issn.2095-5227.2019.11.002.孟三彦, 丁广成, 时昌立, 等. 立体定向放疗联合化疗治疗局部晚期肝门胆管癌疗效观察[J]. 解放军医学院学报, 2019, 40( 11): 1014- 1017, 1033. DOI: 10.3969/j.issn.2095-5227.2019.11.002. [32] MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age[J]. Nature, 2011, 480( 7378): 480- 489. DOI: 10.1038/nature10673. [33] POSTOW MA, CALLAHAN MK, BARKER CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma[J]. N Engl J Med, 2012, 366( 10): 925- 931. DOI: 10.1056/nejmoa1112824. [34] YOON SB, WOO SM, CHUN JW, et al. The predictive value of PD-L1 expression in response to anti-PD-1/PD-L1 therapy for biliary tract cancer: A systematic review and meta-analysis[J]. Front Immunol, 2024, 15: 1321813. DOI: 10.3389/fimmu.2024.1321813. [35] TOMLINSON JL, VALLE JW, ILYAS SI. Immunobiology of cholangiocarcinoma[J]. J Hepatol, 2023, 79( 3): 867- 875. DOI: 10.1016/j.jhep.2023.05.010. [36] PIHA-PAUL SA, OH DY, UENO M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies[J]. Int J Cancer, 2020, 147( 8): 2190- 2198. DOI: 10.1002/ijc.33013. [37] KIM RD, CHUNG V, ALESE OB, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer[J]. JAMA Oncol, 2020, 6( 6): 888. DOI: 10.1001/jamaoncol.2020.0930. [38] ABOU-ALFA GK, SAHAI V, HOLLEBECQUE A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study[J]. Lancet Oncol, 2020, 21( 5): 671- 684. DOI: 10.1016/s1470-2045(20)30109-1. [39] OHBA A, MORIZANE C, KAWAMOTO Y, et al. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-expressing biliary tract cancer(HERB; NCCH1805): A multicenter, single-arm, phase Ⅱ trial[J]. J Clin Oncol, 2024, 42( 27): 3207- 3217. DOI: 10.1200/jco.23.02010. [40] JAVLE M, BORAD MJ, AZAD NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer(MyPathway): A multicentre, open-label, phase 2a, multiple basket study[J]. Lancet Oncol, 2021, 22( 9): 1290- 1300. DOI: 10.1016/s1470-2045(21)00336-3. [41] HARDING JJ, FAN J, OH DY, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer(HERIZON-BTC-01): A multicentre, single-arm, phase 2b study[J]. Lancet Oncol, 2023, 24( 7): 772- 782. DOI: 10.1016/s1470-2045(23)00242-5. [42] OH DY, RUTH HE A, QIN SK, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer[J]. NEJM Evid, 2022, 1( 8). DOI: 10.1056/evidoa2200015. [43] KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/s0140-6736(23)00727-4. [44] SHI GM, HUANG XY, MA L, et al. First-line tislelizumab and ociperlimab combined with gemcitabine and cisplatin in advanced biliary tract cancer(ZSAB-TOP): A multicenter, single-arm, phase 2 study[J]. Sig Transduct Target Ther, 2025, 10: 260. DOI: 10.1038/s41392-025-02356-y. [45] SHI GM, HUANG XY, WU D, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study[J]. Sig Transduct Target Ther, 2023, 8: 106. DOI: 10.1038/s41392-023-01317-7. -



 PDF下载 ( 740 KB)
PDF下载 ( 740 KB)

 下载:
下载:


