多学科管理模式对门静脉高压患者预后的影响
DOI: 10.12449/JCH251017
Effect of the multidisciplinary management model on the prognosis of patients with portal hypertension
-
摘要:
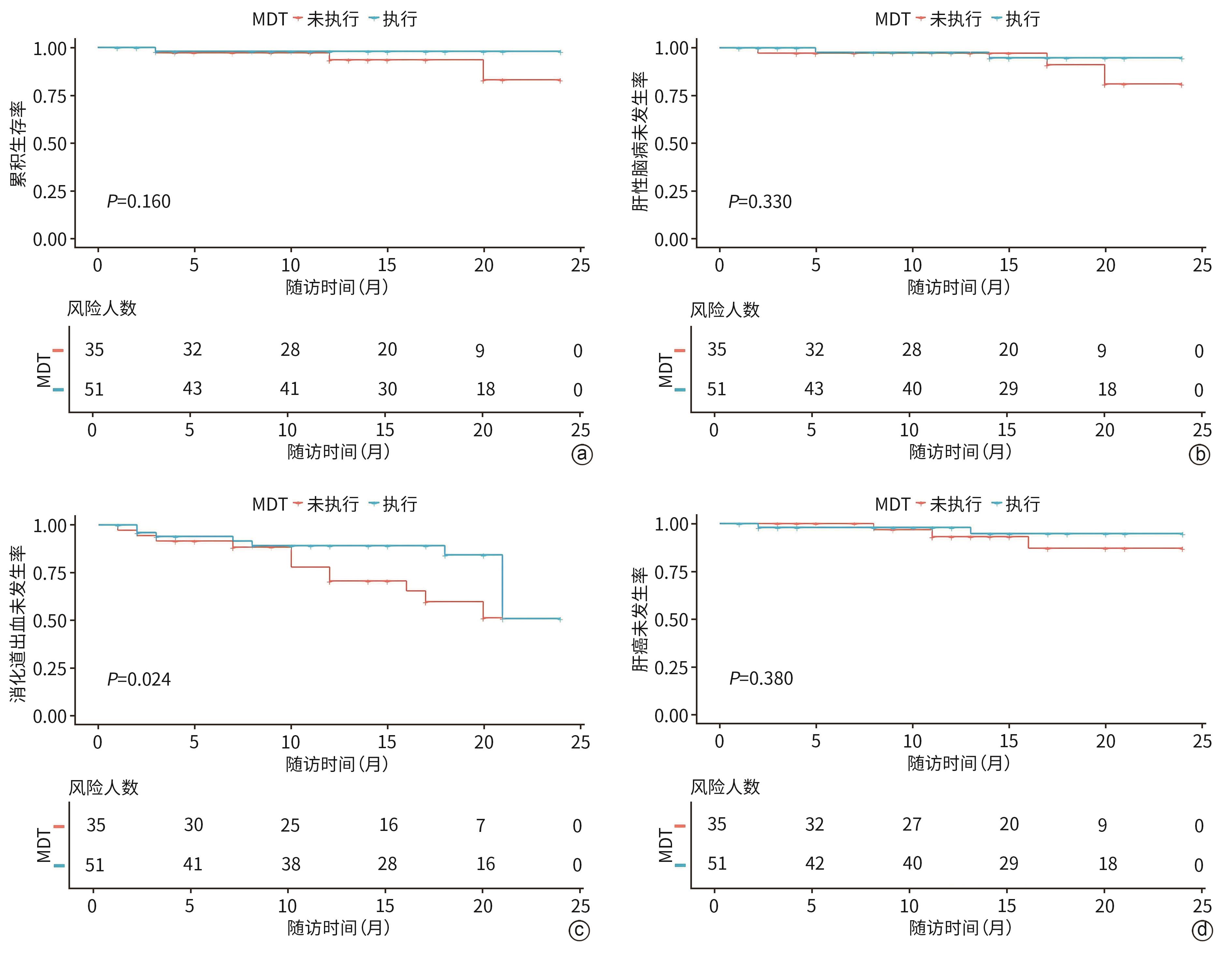
目的 评估多学科团队(MDT)管理模式在改善肝硬化门静脉高压患者预后中的效果。 方法 纳入深圳市第三人民医院2022年5月—2024年7月入院的肝硬化门静脉高压患者86例,按照是否执行MDT治疗方案分为执行组(n=51)和未执行组(n=35)。收集患者基线临床资料,从入院到随访结束(随访截止时间为2025年1月),观察消化道出血、肝性脑病、肝癌、死亡结局发生情况。正态分布的计量资料两组间比较采用成组t检验;非正态分布的计量资料两组间比较采用Wilcoxon秩和检验。计数资料两组间比较采用χ2检验或Fisher精确检验。通过Kaplan-Meier法绘制生存曲线描述终点事件(消化道出血、肝性脑病、肝癌以及死亡)的累积发生率,采用Log-rank检验比较组间差异。Cox比例风险回归模型分析MDT管理对患者预后的影响。 结果 执行组与未执行组基线门静脉内径(t=1.216,P=0.017)、腹水(χ2=4.515,P=0.034)差异有统计学意义。所有患者平均随访(14.6±6.2)个月,生存曲线分析结果显示,两组消化道出血累积发生率差异有统计学意义(χ2=4.573,P=0.024),其余终点事件发生率差异均无统计学意义(P值均>0.05)。Cox回归分析显示,执行组消化道出血风险降低(HR=0.262,95%CI:0.110~0.630,P=0.003)。 结论 执行MDT治疗方案可显著降低肝硬化门静脉高压患者短期消化道出血风险,其长期效益需进一步随访验证。 Abstract:Objective To investigate the effect of the multidisciplinary team (MDT) management model in improving the prognosis of patients with cirrhotic portal hypertension. Methods A total of 86 patients with cirrhotic portal hypertension who were admitted to Shenzhen Third People’s Hospital from May 2022 to July 2024 were enrolled, and according to whether the MDT treatment regimen was implemented, they were divided into execution group with 51 patients and non-execution group with 35 patients. Baseline clinical data were collected, and the patients were observed in terms of gastrointestinal bleeding, hepatic encephalopathy, liver cancer, and death from admission to the end of follow-up (January 2025). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups. The Kaplan-Meier method was used to plot survival curves for the cumulative incidence rates of endpoint events (gastrointestinal bleeding, hepatic encephalopathy, liver cancer, and death), and the Log-rank test was used for comparison between groups. The Cox proportional-hazards regression model analysis was used to investigate the effect of MDT management on the prognosis of patients. Results There were significant differences between the execution group and the non-execution group in diameter of the portal vein (t=1.216, P=0.017) and ascites (χ2=4.515, P=0.034) at baseline. The patients were followed up for 14.6±6.2 months, and the survival curve analysis showed that there was a significant difference in the cumulative incidence rate of gastrointestinal bleeding between the two groups (χ2=4.573, P=0.024), while there were no significant differences in the incidence rates of other outcome events between the two groups (all P>0.05). The Cox regression analysis showed that the execution group had a reduced risk of gastrointestinal bleeding (hazard ratio=0.262, 95% confidence interval: 0.110 — 0.630, P=0.003). Conclusion Implementation of the MDT treatment regimen can significantly reduce the short-term risk of gastrointestinal bleeding in patients with cirrhotic portal hypertension, while its long-term benefits require further follow-up verification. -
表 1 两组患者基线特征比较
Table 1. Baseline characteristics of two groups of patients
项目 未执行组(n=35) 执行组(n=51) 统计值 P值 年龄(岁) 52.0(42.5~62.5) 52.0(42.0~58.5) Z=-0.603 0.547 BMI(kg/m2) 23.5±3.9 23.6±3.4 t=-0.147 0.715 WBC(×109/L) 2.7(2.3~4.2) 3.0(2.2~4.5) Z=-0.123 0.902 Hb(g/L) 99.0(73.5~123.0) 113.0(88.0~127.0) Z=-1.508 0.132 PLT(×109/L) 56.0(39.5~72.0) 58.0(44.0~81.5) Z=-0.967 0.334 HBA1C(%) 5.5(5.2~6.8) 5.4(5.0~6.3) Z=-0.742 0.458 PT(s) 16.4±2.7 16.2±2.4 t=0.356 0.765 TBil(μmol/L) 20.5(12.6~28.7) 23.9(14.9~33.5) Z=-1.270 0.204 TBA(μmol/L) 25.6(16.4~53.8) 28.1(13.5~54.2) Z=-0.270 0.787 ALT(U/L) 22.0(17.5~40.8) 24.0(19.0~39.0) Z=-0.559 0.576 AST(U/L) 34.0(22.0~45.0) 34.0(26.0~45.5) Z=-0.800 0.424 ALP(U/L) 101.8±45.4 100.2±46.9 t=0.158 0.826 GGT(U/L) 47.0(28.5~81.5) 39.0(25.5~76.5) Z=-0.668 0.504 Alb(g/L) 35.9(32.5~39.1) 36.4(32.8~40.2) Z=-0.985 0.325 GLU(μmol/L) 5.1(4.8~6.6) 5.7(4.9~6.5) Z=-0.335 0.738 UREA(mmol/L) 4.5(3.7~6.5) 4.4(3.8~6.4) Z=-0.053 0.958 Cr(μmol/L) 72.0(61.0~93.0) 67.0(55.0~83.5) Z=-1.266 0.205 CRP(mg/L) 2.7(0.7~6.9) 1.4(0.6~2.5) Z=-1.705 0.088 PCT(ng/mL) 0.1(0.1~0.1) 0.1(0.0~0.1) Z=-1.334 0.182 CHO(mmol/L) 3.4(2.7~4.3) 3.6(2.9~4.4) Z=-0.652 0.515 TG(mmol/L) 1.0±0.4 0.9±0.3 t=0.254 0.782 AFP(ng/mL) 1.9(1.7~3.1) 2.8(1.8~5.6) Z=-1.440 0.150 HBsAg(IU/mL) 180.0(8.0~511.0) 143.5(0.4~737.8) Z=-0.326 0.744 HBeAg(COI) 0.1(0.1~0.1) 0.1(0.1~0.2) Z=-0.262 0.793 门静脉流速(cm/s) 16.7±3.3 16.6±3.7 t=0.102 0.972 脾厚度(mm) 56.0(49.0~63.0) 51.0(45.0~58.0) Z=-1.410 0.159 脾长度(mm) 163.0(142.0~181.0) 150.5(133.5~169.5) Z=-1.442 0.149 门静脉内径(mm) 14.5±2.1 13.8±3.2 t=1.216 0.017 LSM(kPa) 13.2±5.0 13.5±2.6 t=-0.155 0.519 HVPG(mmHg) 14.0(12.0~18.0) 13.5(12.0~17.0) Z=-0.702 0.621 Child-Pugh评分(分) 7.1±1.7 6.7±1.6 t=1.158 0.186 MELD评分(分) 10.0(8.0~12.0) 9.0(7.0~11.0) Z=1.320 0.187 曲张静脉最大内径(mm) 10.0(6.0~15.0) 15.0(8.0~20.0) Z=1.562 0.119 随访时间(月) 15.0(12.0~20.0) 17.0(11.0~20.0) Z=1.520 0.128 静脉曲张情况[例(%)]1) χ2=1.515 0.218 否 2(6.1) 7(14.9) 是 31(93.9) 40(85.1) 红色征[例(%)] χ2=2.473 0.116 阴性 5(16.1) 13(32.5) 阳性 26(83.9) 27(67.5) 性别[例(%)] χ2=3.219 0.073 女 4(11.4) 14(27.5) 男 31(88.6) 37(72.5) 饮酒[例(%)] χ2=0.959 0.366 否 26(74.3) 42(82.4) 是 9(25.7) 9(17.6) 合并糖尿病[例(%)] χ2=0.934 0.334 否 25(71.4) 41(80.4) 是 10(28.6) 10(19.6) 合并心血管疾病[例(%)] χ2=0.002 0.962 否 31(88.6) 45(88.2) 是 4(11.4) 6(11.8) 病因[例(%)] χ2=4.835 0.680 乙型肝炎 22(62.9) 33(64.7) 丙型肝炎 0(0.0) 2(3.9) 酒精性 2(5.7) 2(3.9) 自身免疫性 0(0.0) 3(5.9) 药物性 1(2.9) 2(3.9) 不明原因 5(14.3) 4(7.8) 肝豆状核变性 1(2.9) 1(2.0) 乙型肝炎+酒精 4(11.4) 4(7.8) HBV DNA[例(%)] χ2=0.055 0.820 阴性 19(73.1) 28(75.7) <103 IU/mL 7(26.9) 7(18.9) 103~105 IU/mL 0(0.0) 1(2.7) >105 IU/mL 0(0.0) 1(2.7) 腹水[例(%)] χ2=4.515 0.034 否 16(45.7) 35(68.6) 是 19(54.3) 16(31.4) 乙型肝炎抗病毒治疗[例(%)] χ2=1.963 0.743 否 1(3.8) 2(5.4) 恩替卡韦 17(65.4) 23(62.2) 丙酚替诺福韦 6(23.1) 9(24.3) 替诺福韦 1(3.8) 3(8.1) 艾米替诺福韦 1(3.8) 0(0.0) 注:1)6例患者未能明确静脉曲张情况,其中MDT未执行组2例,因患者未通过麻醉风险评估,不能做胃镜检查;MDT执行组4例,其中2例患者主诉近期在当地医院做胃镜检查,但未见具体报告,1例不同意行胃镜检查,余1例患者未通过麻醉风险评估。
表 2 两组患者终点事件发生情况比较
Table 2. Comparison of endpoint events incidence between two groups of patients
终点事件 未执行组(n=35) 执行组(n=51) χ2值 P值 消化道出血[例(%)] 12(34.3) 8(15.7) 4.283 0.045 肝性脑病[例(%)] 3(8.6) 2(3.9) 0.819 0.393 肝癌[例(%)] 3(8.6) 2(3.9) 0.819 0.393 死亡[例(%)] 3(8.6) 1(2.0) 2.045 0.300 表 3 MDT治疗方案实施对消化道出血影响的Cox回归分析
Table 3. Cox regression analysis of MDT implementation on gastrointestinal bleeding occurrence
变量 β值 SE Wald P值 HR(95%CI) 模型1 -0.916 0.432 4.50 0.032 0.400(0.101~0.902) 模型2 -1.204 0.440 7.49 0.030 0.300(0.103~0.901) 模型3 -1.204 0.440 7.49 0.027 0.300(0.101~0.902) 模型4 -1.352 0.455 8.89 0.003 0.262(0.110~0.630) 注:模型1,校正性别、年龄;模型2,校正性别、年龄、静脉曲张内径、红色征;模型3,校正性别、年龄、静脉曲张内径、红色征、HVPG;模型4,校正性别、年龄、静脉曲张内径、红色征、HVPG、腹水、门静脉内径。
-
[1] Beijing Society of Portal Hypertension, Beijing Medical Association; Portal Hypertension Expert Committee, Liver Disease Committee of Chinese Research Hospital Association; Liver Disease Committee of Chinese Research Hospital Association. Expert consensus on multidisciplinary diagnosis and treatment of cirrhotic portal hypertension(based on hepatic venous pressure gradient)[J]. J Clin Hepatol, 2021, 37( 9): 2037- 2044. DOI: 10.3969/j.issn.1001-5256.2021.09.008.北京医师协会门静脉高压专科医师分会, 中国研究型医院学会肝病专业委员会门静脉高压学组, 中国研究型医院学会肝病专业委员会. 肝硬化门静脉高压症多学科诊治(基于肝静脉压力梯度)专家共识[J]. 临床肝胆病杂志, 2021, 37( 9): 2037- 2044. DOI: 10.3969/j.issn.1001-5256.2021.09.008. [2] European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69( 2): 406- 460. DOI: 10.1016/j.jhep.2018.03.024. [3] BROWN RS Jr, BROWN KA, FLAMM S, et al. Screening and management of portal hypertension and varices in cirrhosis: Expert perspectives[J]. Hepatol Commun, 2025, 9( 4): e0682. DOI: 10.1097/HC9.0000000000000682. [4] AITHAL GP, PALANIYAPPAN N, CHINA L, et al. Guidelines on the management of ascites in cirrhosis[J]. Gut, 2021, 70( 1): 9- 29. DOI: 10.1136/gutjnl-2020-321790. [5] Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on the diagnosis and treatment of esophageal and gastric variceal rupture bleeding in cirrhotic portal hypertension(2025 edition)[J]. Chin J Dig Surg, 2025, 24( 3): 271- 280. DOI: 10.3760/cma.j.cn115610-20241228-00590.中华医学会外科学分会脾及门静脉高压外科学组. 肝硬化门静脉高压症食管、胃底静脉曲张破裂出血诊治专家共识(2025版)[J]. 中华消化外科杂志, 2025, 24( 3): 271- 280. DOI: 10.3760/cma.j.cn115610-20241228-00590. [6] KAPLAN DE, RIPOLL C, THIELE M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis[J]. Hepatology, 2024, 79( 5): 1180- 1211. DOI: 10.1097/HEP.0000000000000647. [7] Chinese Society of Hepatology, Chinese Society of Gastroenterology, and Chinese Society of Digestive Endoscopology of Chinese Medical Association. Guidelines on the management of esophagogastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2023, 39( 3): 527- 538.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会消化内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2023, 39( 3): 527- 538. [8] de FRANCHIS R, BOSCH J, GARCIA-TSAO G, et al. Baveno VII- Renewing consensus in portal hypertension[J]. J Hepatol, 2022, 76( 4): 959- 974. DOI: 10.1016/j.jhep.2021.12.022. [9] JINDAL A, BHARDWAJ A, KUMAR G, et al. Clinical decompensation and outcomes in patients with compensated cirrhosis and a hepatic venous pressure gradient ≥20 mm Hg[J]. Am J Gastroenterol, 2020, 115( 10): 1624- 1633. DOI: 10.14309/ajg.0000000000000653. [10] TSENG Y, MA L, LV M, et al. The role of a multidisciplinary team in the management of portal hypertension[J]. BMC Gastroenterol, 2020, 20( 1): 83. DOI: 10.1186/s12876-020-01203-4. [11] LIU Z, SUO C, MAO X, et al. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017[J]. Cancer, 2020, 126( 10): 2267- 2278. DOI: 10.1002/cncr.32789. [12] SHI K, ZHANG Q, LI YX, et al. Research advances in the risk prediction models for chronic hepatitis B-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36( 10): 2315- 2319. DOI: 10.3969/j.issn.1001-5256.2020.10.034.时克, 张群, 李玉鑫, 等. 慢性乙型肝炎相关肝细胞癌风险预测模型的研究进展[J]. 临床肝胆病杂志, 2020, 36( 10): 2315- 2319. DOI: 10.3969/j.issn.1001-5256.2020.10.034. [13] CHINA L, TITTANEGRO T, CROCOMBE D, et al. Investigating potential confounding by indication when considering the association between proton pump inhibitor use, infection, hepatic encephalopathy and mortality in hospitalised decompensated cirrhosis: A post-hoc analysis of the ATTIRE trial[J]. EClinicalMedicine, 2023, 58: 101924. DOI: 10.1016/j.eclinm.2023.101924. [14] SANO T, AMANO K, IDE T, et al. A combination of hepatic encephalopathy and body mass index was associated with the point of no return for improving liver functional reserve after sofosbuvir/velpatasvir treatment in patients with hepatitis C virus-related decompensated cirrhosis[J]. Hepatol Res, 2023, 53( 1): 26- 34. DOI: 10.1111/hepr.13837. [15] LIN Y, LI JF, LI FY, et al. Role of neuroimmune communication via the gut-brain axis in the pathogenesis of hepatic encephalopathy[J]. J Clin Hepatol, 2024, 40( 12): 2518- 2523. DOI: 10.12449/JCH241224.林镛, 李炯汾, 李飞燕, 等. 肠-脑轴神经免疫通信在肝性脑病发病机制中的作用[J]. 临床肝胆病杂志, 2024, 40( 12): 2518- 2523. DOI: 10.12449/JCH241224. [16] NARDELLI S, GIOIA S, FACCIOLI J, et al. Hepatic encephalopathy- recent advances in treatment and diagnosis[J]. Expert Rev Gastroenterol Hepatol, 2023, 17( 3): 225- 235. DOI: 10.1080/17474124.2023.2183386. -



 PDF下载 ( 1060 KB)
PDF下载 ( 1060 KB)

 下载:
下载:


