胆管细胞衰老在胆汁淤积性肝病中的作用机制及其靶向治疗进展
DOI: 10.12449/JCH250836
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:徐华明、刘延鑫负责课题设计,资料分析,拟定写作思路;杨柳、徐华明负责撰写和修改论文;郑思嘉、杨念、闫五玲参与收集数据,核对最后定稿和参考文献;杨柳、徐华明负责图片绘制。
Mechanism of action of cholangiocyte senescence in cholestatic liver disease and retated targeted therapies
-
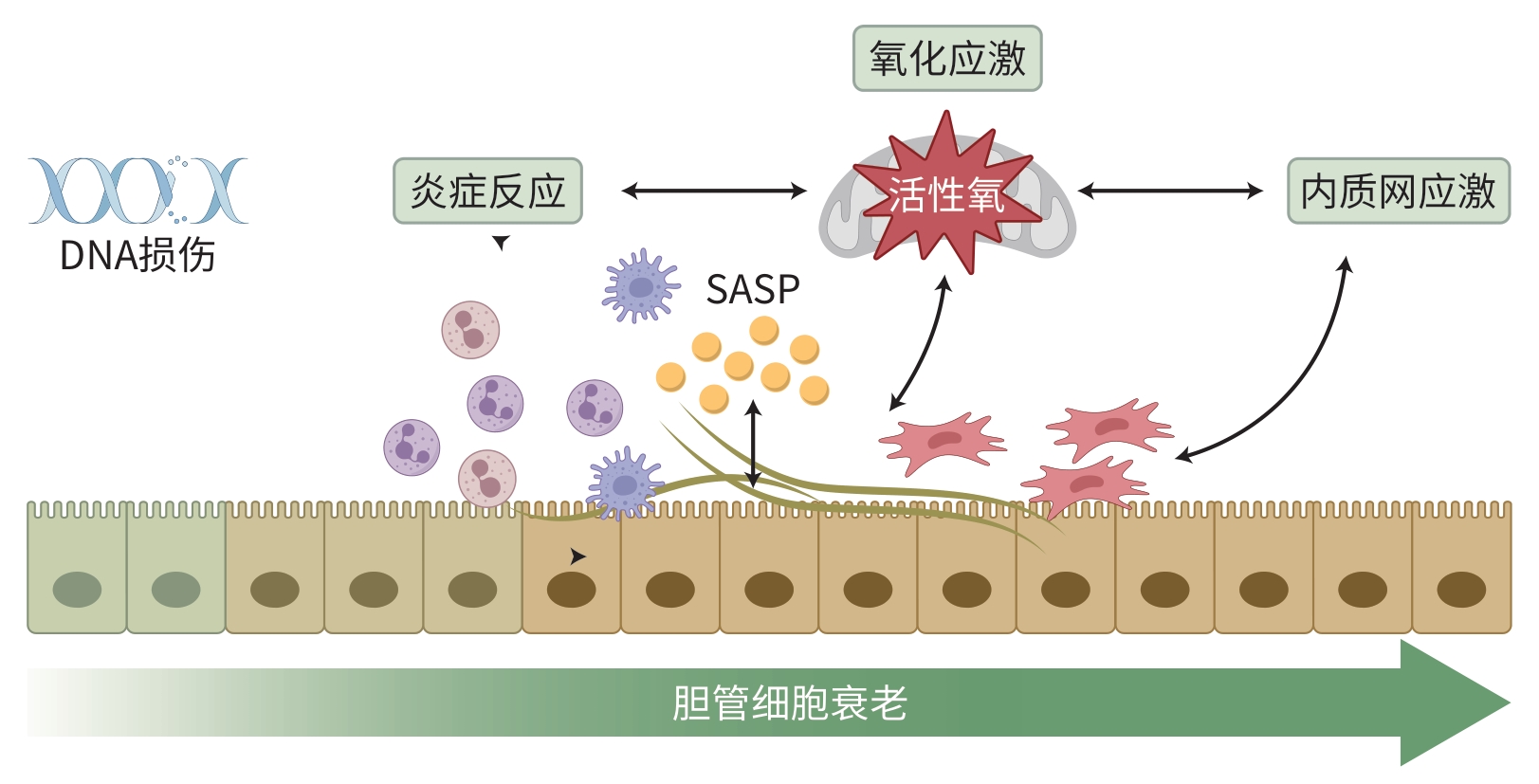
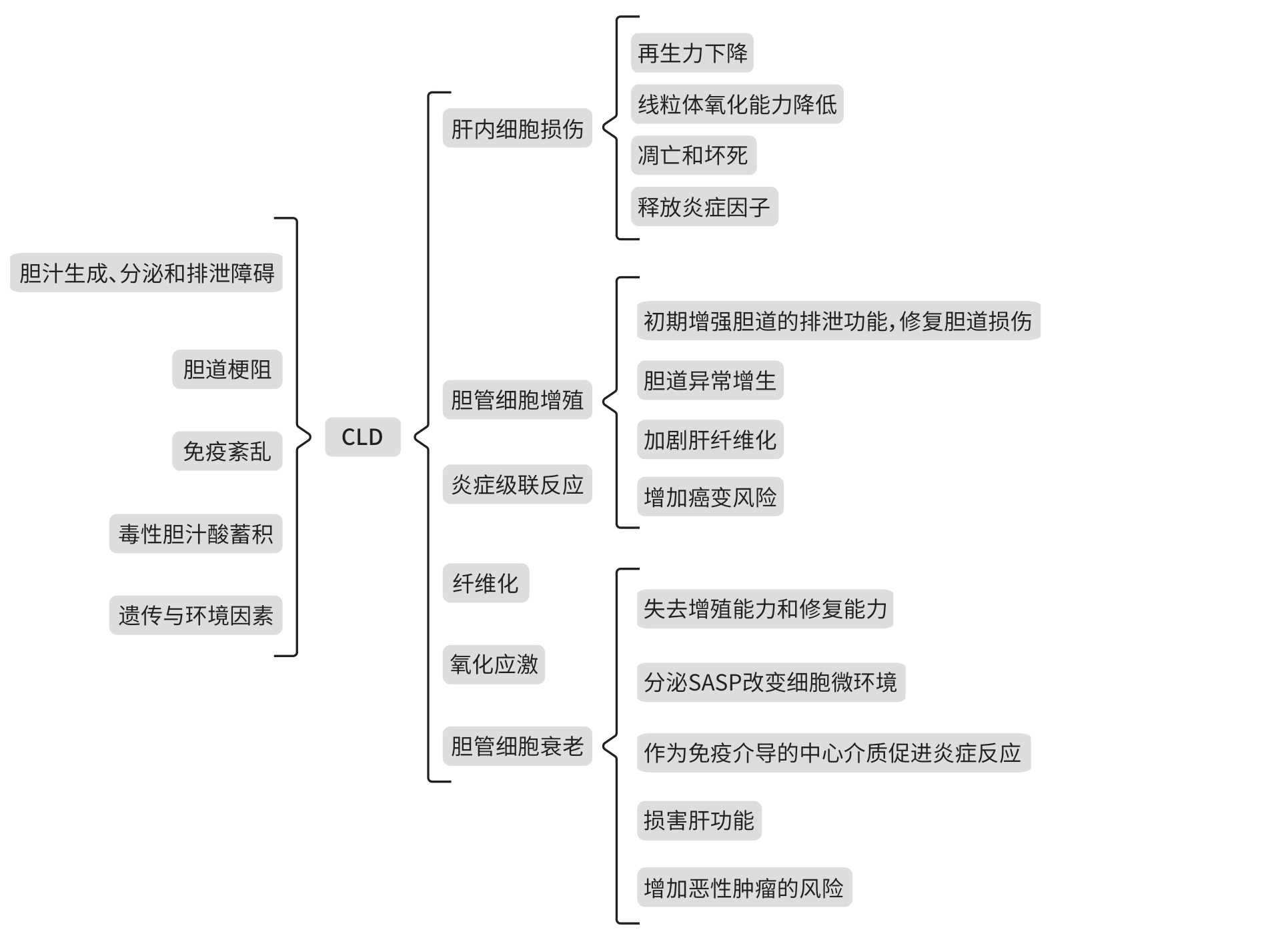
摘要: 胆汁淤积性肝病(CLD)是由各种原因导致胆汁酸分泌和代谢障碍引起的肝脏病变,表现出许多慢性肝系疾病的共同病理特征。近年来,胆管细胞衰老(CS)在CLD发病过程中的作用越来越受到关注,不仅参与其发生和进展,而且与进程和预后显著相关。靶向清除胆管衰老细胞或阻断衰老相关通路可改善CLD。本文针对CS在CLD中的作用和影响因素、CLD现有研究进展进行归纳总结和探讨,以期为后续CLD的研究提供理论参考。Abstract: Cholestatic liver disease (CLD) is a liver condition caused by disorders in bile acid secretion and metabolism due to various reasons, and it has the common pathological features of various chronic liver diseases. In recent years, the role of cholangiocyte senescence (CS) in the pathogenesis of CLD has attracted more and more attention, and CS not only participates in the development and progression of CLD, but it is also significantly associated with the course and prognosis of the disease. Targeted clearance of senescent cholangiocytes or blocking senescence-related pathways can improve CLD. This article summarizes the role of CS in CLD, related influencing factors, and the research advances in CLD, in order to provide a theoretical reference for subsequent studies on CLD.
-
Key words:
- Cholestasis /
- Cholangiocytes /
- Cellular Senescence
-
[1] TRIVEDI PJ, HIRSCHFIELD GM, ADAMS DH, et al. Immunopathogenesis of primary biliary cholangitis, primary sclerosing cholangitis and autoimmune hepatitis: Themes and concepts[J]. Gastroenterology, 2024, 166( 6): 995- 1019. DOI: 10.1053/j.gastro.2024.01.049. [2] PARK JW, KIM JH, KIM SE, et al. Primary biliary cholangitis and primary sclerosing cholangitis: Current knowledge of pathogenesis and therapeutics[J]. Biomedicines, 2022, 10( 6): 1288. DOI: 10.3390/biomedicines10061288. [3] TANAKA A. New therapies on the horizon for primary biliary cholangitis[J]. Drugs, 2024, 84( 1): 1- 15. DOI: 10.1007/s40265-023-01979-1. [4] LEVY C, MANNS M, HIRSCHFIELD G. New treatment paradigms in primary biliary cholangitis[J]. Clin Gastroenterol Hepatol, 2023, 21( 8): 2076- 2087. DOI: 10.1016/j.cgh.2023.02.005. [5] FIORUCCI S, URBANI G, DI GIORGIO C, et al. Bile acids-based therapies for primary sclerosing cholangitis: Current landscape and future developments[J]. Cells, 2024, 13( 19): 1650. DOI: 10.3390/cells13191650. [6] LUO X, LU LG. Progress in the management of patients with cholestatic liver disease: Where are we and where are we going?[J]. J Clin Transl Hepatol, 2024, 12( 6): 581- 588. DOI: 10.14218/JCTH.2023.00519. [7] CAI XR, TACKE F, GUILLOT A, et al. Cholangiokines: Undervalued modulators in the hepatic microenvironment[J]. Front Immunol, 2023, 14: 1192840. DOI: 10.3389/fimmu.2023.1192840. [8] CHUNG BK, KARLSEN TH, FOLSERAAS T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864( 4 Pt B): 1390- 1400. DOI: 10.1016/j.bbadis.2017.08.020. [9] TRUSSONI CE, O’HARA SP, LARUSSO NF. Cellular senescence in the cholangiopathies: A driver of immunopathology and a novel therapeutic target[J]. Semin Immunopathol, 2022, 44( 4): 527- 544. DOI: 10.1007/s00281-022-00909-9. [10] JALAN-SAKRIKAR N, GUICCIARDI ME, O’HARA SP, et al. Central role for cholangiocyte pathobiology in cholestatic liver diseases[J]. Hepatology, 2024: 10.1097/HEP. 0000000000001093. DOI: 10.1097/HEP.0000000000001093. [11] WEI WQ, JI SP. Cellular senescence: Molecular mechanisms and pathogenicity[J]. J Cell Physiol, 2018, 233( 12): 9121- 9135. DOI: 10.1002/jcp.26956. [12] OGRODNIK M, ACOSTA JC, ADAMS PD, et al. Guidelines for minimal information on cellular senescence experimentation in vivo[J]. Cell, 2024, 187( 16): 4150- 4175. DOI: 10.1016/j.cell.2024.05.059. [13] ZHANG YM, HUANG SY, XIE B, et al. Aging, cellular senescence, and glaucoma[J]. Aging Dis, 2024, 15( 2): 546- 564. DOI: 10.14336/AD.2023.0630-1. [14] CAZZAGON N, SARCOGNATO S, FLOREANI A, et al. Cholangiocyte senescence in primary sclerosing cholangitis is associated with disease severity and prognosis[J]. JHEP Rep, 2021, 3( 3): 100286. DOI: 10.1016/j.jhepr.2021.100286. [15] BARRON-MILLAR B, OGLE L, MELLS G, et al. The serum proteome and ursodeoxycholic acid response in primary biliary cholangitis[J]. Hepatology, 2021, 74( 6): 3269- 3283. DOI: 10.1002/hep.32011. [16] SASAKI M, SATO Y, NAKANUMA Y. Increased p16INK4a-expressing senescent bile ductular cells are associated with inadequate response to ursodeoxycholic acid in primary biliary cholangitis[J]. J Autoimmun, 2020, 107: 102377. DOI: 10.1016/j.jaut.2019.102377. [17] KYRITSI K, KENNEDY L, MEADOWS V, et al. Mast cells induce ductular reaction mimicking liver injury in mice through mast cell-derived transforming growth factor beta 1 signaling[J]. Hepatology, 2021, 73( 6): 2397- 2410. DOI: 10.1002/hep.31497. [18] WAN Y, ZHOU TH, SLEVIN E, et al. Liver-specific deletion of microRNA-34a alleviates ductular reaction and liver fibrosis during experimental cholestasis[J]. FASEB J, 2023, 37( 2): e22731. DOI: 10.1096/fj.202201453R. [19] KOSAR K, CORNUET P, SINGH S, et al. WNT7B regulates cholangiocyte proliferation and function during murine cholestasis[J]. Hepatol Commun, 2021, 5( 12): 2019- 2034. DOI: 10.1002/hep4.1784. [20] RONCA V, MANCUSO C, MILANI C, et al. Immune system and cholangiocytes: A puzzling affair in primary biliary cholangitis[J]. J Leukoc Biol, 2020, 108( 2): 659- 671. DOI: 10.1002/JLB.5MR0320-200R. [21] LAN T, QIAN SJ, TANG CW, et al. Role of immune cells in biliary repair[J]. Front Immunol, 2022, 13: 866040. DOI: 10.3389/fimmu.2022.866040. [22] SASAKI M, MIYAKOSHI M, SATO Y, et al. Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis[J]. J Hepatol, 2010, 53( 2): 318- 325. DOI: 10.1016/j.jhep.2010.03.008. [23] ZHANG L, PITCHER LE, PRAHALAD V, et al. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics[J]. FEBS J, 2023, 290( 5): 1362- 1383. DOI: 10.1111/febs.16350. [24] WU N, MENG FY, INVERNIZZI P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice[J]. Hepatology, 2016, 64( 3): 865- 879. DOI: 10.1002/hep.28622. [25] FERREIRA-GONZALEZ S, LU WY, RAVEN A, et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration[J]. Nat Commun, 2018, 9( 1): 1020. DOI: 10.1038/s41467-018-03299-5. [26] KIOURTIS C, TERRADAS-TERRADAS M, GEE LM, et al. Hepatocellular senescence induces multi-organ senescence and dysfunction via TGFβ[J]. Nat Cell Biol, 2024, 26( 12): 2075- 2083. DOI: 10.1038/s41556-024-01543-3. [27] YANG Y, WANG JL, WAN JH, et al. PTEN deficiency induces an extrahepatic cholangitis-cholangiocarcinoma continuum via aurora kinase A in mice[J]. J Hepatol, 2024, 81( 1): 120- 134. DOI: 10.1016/j.jhep.2024.02.018. [28] SHANG DS, SUN DL, SHI CY, et al. Activation of epidermal growth factor receptor signaling mediates cellular senescence induced by certain pro-inflammatory cytokines[J]. Aging Cell, 2020, 19( 5): e13145. DOI: 10.1111/acel.13145. [29] LU XY, LIU L. Genome stability from the perspective of telomere length[J]. Trends Genet, 2024, 40( 2): 175- 186. DOI: 10.1016/j.tig.2023.10.013. [30] SHIM HS, IACONELLI J, SHANG XY, et al. TERT activation targets DNA methylation and multiple aging hallmarks[J]. Cell, 2024, 187( 15): 4030- 4042. e 13. DOI: 10.1016/j.cell.2024.05.048. [31] JALAN-SAKRIKAR N, ANWAR A, YAQOOB U, et al. Telomere dysfunction promotes cholangiocyte senescence and biliary fibrosis in primary sclerosing cholangitis[J]. JCI Insight, 2023, 8( 20): e170320. DOI: 10.1172/jci.insight.170320. [32] YANG L, ZHENG SJ, YANG N, et al. Research progress of inflammatory response and oxidative stress mechanism of cholestatic liver disease and intervention progress of Chinese medicine[J/OL]. China J Chin Med, 1- 10[ 2024-12-29]. http://kns.cnki.net/kcms/detail/41.1411.R.20240929.1140.006.html. http: //kns.cnki.net/kcms/detail/41.1411.R.20240929.1140.006.html杨柳, 郑思嘉, 杨念, 等. 胆汁淤积肝病的炎症反应和氧化应激机制及中医药干预研究[J/OL]. 中医学报, 1- 10[ 2024-12-29]. http://kns.cnki.net/kcms/detail/41.1411.R.20240929.1140.006.html. http: //kns.cnki.net/kcms/detail/41.1411.R.20240929.1140.006.html [33] von ZGLINICKI T. Oxidative stress and cell senescence as drivers of ageing: Chicken and egg[J]. Ageing Res Rev, 2024, 102: 102558. DOI: 10.1016/j.arr.2024.102558. [34] FLOREANI A, GABBIA D, de MARTIN S. Primary biliary cholangitis: Primary autoimmune disease or primary secretory defect[J]. Expert Rev Gastroenterol Hepatol, 2023, 17( 9): 863- 870. DOI: 10.1080/17474124.2023.2242771. [35] PRIETO J, BANALES JM, MEDINA JF. Primary biliary cholangitis: Pathogenic mechanisms[J]. Curr Opin Gastroenterol, 2021, 37( 2): 91- 98. DOI: 10.1097/MOG.0000000000000703. [36] SASAKI M, SATO Y, NAKANUMA Y. An impaired biliary bicarbonate umbrella may be involved in dysregulated autophagy in primary biliary cholangitis[J]. Lab Invest, 2018, 98( 6): 745- 754. DOI: 10.1038/s41374-018-0045-4. [37] LI Q, LIN Y, LIANG GY, et al. Autophagy and senescence: The molecular mechanisms and implications in liver diseases[J]. Int J Mol Sci, 2023, 24( 23): 16880. DOI: 10.3390/ijms242316880. [38] SASAKI M, MIYAKOSHI M, SATO Y, et al. Autophagy may precede cellular senescence of bile ductular cells in ductular reaction in primary biliary cirrhosis[J]. Dig Dis Sci, 2012, 57( 3): 660- 666. DOI: 10.1007/s10620-011-1929-y. [39] SASAKI M, NAKANUMA Y. Bile acids and deregulated cholangiocyte autophagy in primary biliary cholangitis[J]. Dig Dis, 2017, 35( 3): 210- 216. DOI: 10.1159/000450913. [40] ALSURAIH M, O’HARA SP, WOODRUM JE, et al. Genetic or pharmacological reduction of cholangiocyte senescence improves inflammation and fibrosis in the Mdr2-/- mouse[J]. JHEP Rep, 2021, 3( 3): 100250. DOI: 10.1016/j.jhepr.2021.100250. [41] MAHONEY SA, VENKATASUBRAMANIAN R, DARRAH MA, et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence[J]. Aging Cell, 2024, 23( 3): e14060. DOI: 10.1111/acel.14060. [42] KYRITSI K, FRANCIS H, ZHOU TH, et al. Downregulation of p16 decreases biliary damage and liver fibrosis in the Mdr2-/- mouse model of primary sclerosing cholangitis[J]. Gene Expr, 2020, 20( 2): 89- 103. DOI: 10.3727/105221620X15889714507961. [43] JONES H, HARGROVE L, KENNEDY L, et al. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(-/-) mice[J]. Hepatology, 2016, 64( 4): 1202- 1216. DOI: 10.1002/hep.28704. [44] WANG Y, QIU H, CHEN SP, et al. microRNA-7 deficiency ameliorates d-galactose-induced aging in mice by regulating senescence of Kupffer cells[J]. Aging Cell, 2024, 23( 6): e14145. DOI: 10.1111/acel.14145. [45] JIA RJ, YANG F, YAN PF, et al. Paricalcitol inhibits oxidative stress-induced cell senescence of the bile duct epithelium dependent on modulating Sirt1 pathway in cholestatic mice[J]. Free Radic Biol Med, 2021, 169: 158- 168. DOI: 10.1016/j.freeradbiomed.2021.04.019. [46] KIM JY, KIM SH, SEOK J, et al. Increased PRL-1 in BM-derived MSCs triggers anaerobic metabolism via mitochondria in a cholestatic rat model[J]. Mol Ther Nucleic Acids, 2023, 31: 512- 524. DOI: 10.1016/j.omtn.2023.01.017. [47] TORRES G, SALLADAY-PEREZ IA, DHINGRA A, et al. Genetic origins, regulators, and biomarkers of cellular senescence[J]. Trends Genet, 2024, 40( 12): 1018- 1031. DOI: 10.1016/j.tig.2024.08.007. [48] MONCSEK A, AL-SURAIH MS, TRUSSONI CE, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout(Mdr2-/-) mice[J]. Hepatology, 2018, 67( 1): 247- 259. DOI: 10.1002/hep.29464. [49] YIN YJ, CHEN HH, WANG YZ, et al. Roles of extracellular vesicles in the aging microenvironment and age-related diseases[J]. J Extracell Vesicles, 2021, 10( 12): e12154. DOI: 10.1002/jev2.12154. [50] SURAIH MS AL, TRUSSONI CE, SPLINTER PL, et al. Senescent cholangiocytes release extracellular vesicles that alter target cell phenotype via the epidermal growth factor receptor[J]. Liver Int, 2020, 40( 10): 2455- 2468. DOI: 10.1111/liv.14569. [51] RUDNITSKY E, BRAIMAN A, WOLFSON M, et al. Stem cell-derived extracellular vesicles as senotherapeutics[J]. Ageing Res Rev, 2024, 99: 102391. DOI: 10.1016/j.arr.2024.102391. [52] CHEN WY, ZHU JQ, LIN FY, et al. Human placenta mesenchymal stem cell-derived exosomes delay H2O2 - induced aging in mouse cholangioids[J]. Stem Cell Res Ther, 2021, 12( 1): 201. DOI: 10.1186/s13287-021-02271-3. [53] CHEN LX, ZHOU TH, WHITE T, et al. The apelin-apelin receptor axis triggers cholangiocyte proliferation and liver fibrosis during mouse models of cholestasis[J]. Hepatology, 2021, 73( 6): 2411- 2428. DOI: 10.1002/hep.31545. [54] CECI L, FRANCIS H, ZHOU TH, et al. Knockout of the tachykinin receptor 1 in the Mdr2-/-(Abcb4-/-) mouse model of primary sclerosing cholangitis reduces biliary damage and liver fibrosis[J]. Am J Pathol, 2020, 190( 11): 2251- 2266. DOI: 10.1016/j.ajpath.2020.07.007. [55] GLASER S, GAUDIO E, RENZI A, et al. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice[J]. Am J Physiol Gastrointest Liver Physiol, 2011, 301( 2): G297- G305. DOI: 10.1152/ajpgi.00418.2010. [56] WAN Y, CECI L, WU N, et al. Knockout of α-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes[J]. Lab Invest, 2019, 99( 6): 764- 776. DOI: 10.1038/s41374-018-0178-5. [57] SCHUMACHER JD, GUO GL. Regulation of hepatic stellate cells and fibrogenesis by fibroblast growth factors[J]. Biomed Res Int, 2016, 2016: 8323747. DOI: 10.1155/2016/8323747. [58] WU G, YU F, XIAO Z, et al. Hepatitis B virus X protein downregulates expression of the miR-16 family in malignant hepatocytes in vitro[J]. Br J Cancer, 2011, 105( 1): 146- 153. DOI: 10.1038/bjc.2011.190. [59] O’BRIEN A, ZHOU TH, WHITE T, et al. FGF1 signaling modulates biliary injury and liver fibrosis in the Mdr2-/- mouse model of primary sclerosing cholangitis[J]. Hepatol Commun, 2022, 6( 7): 1574- 1588. DOI: 10.1002/hep4.1909. [60] SASAKI M, SATO Y, NAKANUMA Y. Interferon-induced protein with tetratricopeptide repeats 3 may be a key factor in primary biliary cholangitis[J]. Sci Rep, 2021, 11( 1): 11413. DOI: 10.1038/s41598-021-91016-6. [61] ZHANG WT, LI YL, XIN SY, et al. The emerging roles of IFIT3 in antiviral innate immunity and cellular biology[J]. J Med Virol, 2023, 95( 1): e28259. DOI: 10.1002/jmv.28259. [62] DONG ZN, LUO YH, YUAN ZC, et al. Cellular senescence and SASP in tumor progression and therapeutic opportunities[J]. Mol Cancer, 2024, 23( 1): 181. DOI: 10.1186/s12943-024-02096-7. -



 PDF下载 ( 1160 KB)
PDF下载 ( 1160 KB)

 下载:
下载: