甲胎蛋白阴性肝细胞癌患者肝切除术后预后的危险因素分析及列线图模型的构建
DOI: 10.12449/JCH250820
Risk factors for postoperative prognosis of patients with AFP-negative hepatocellular carcinoma and establishment of a nomogram model
-
摘要:
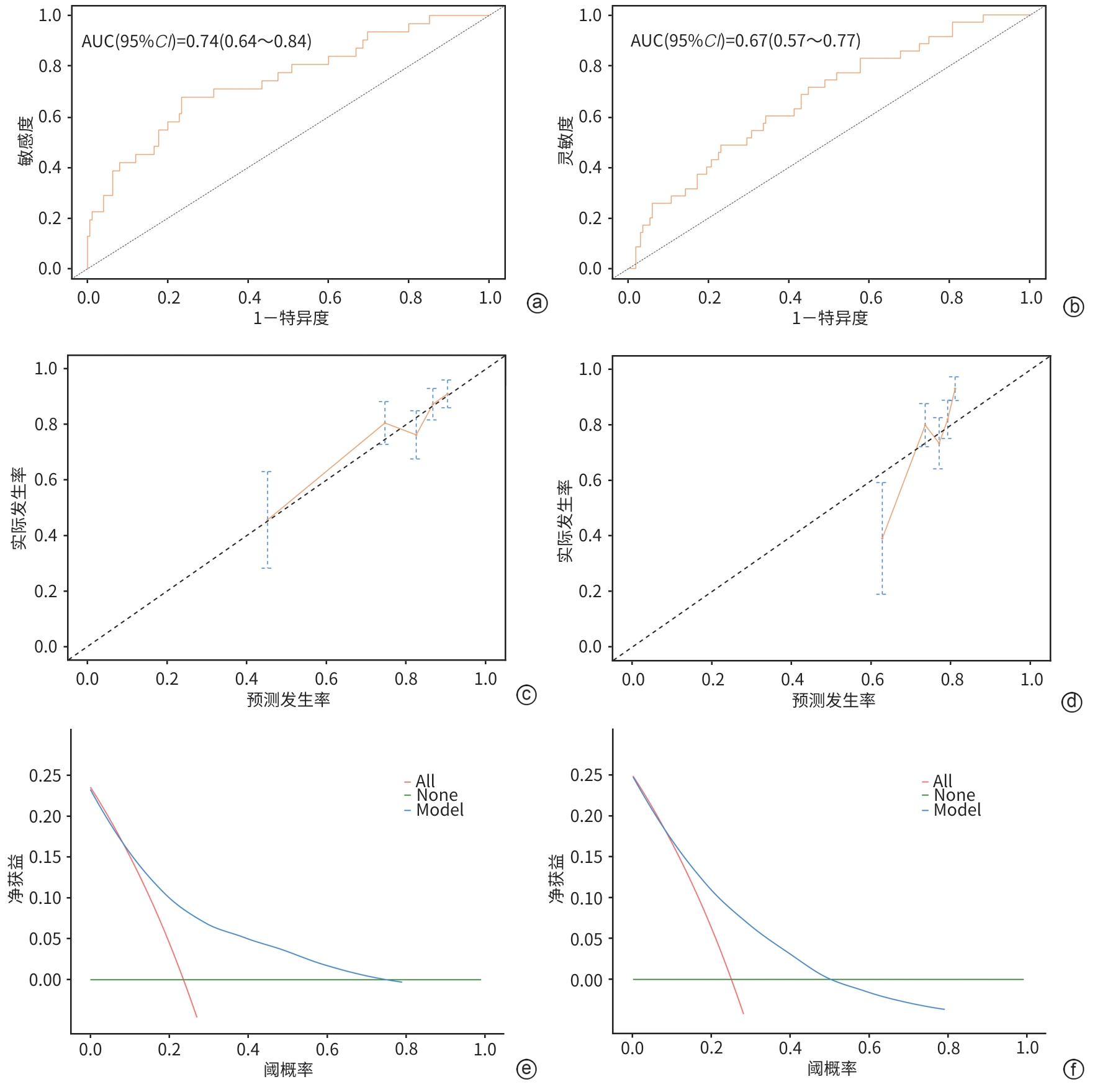
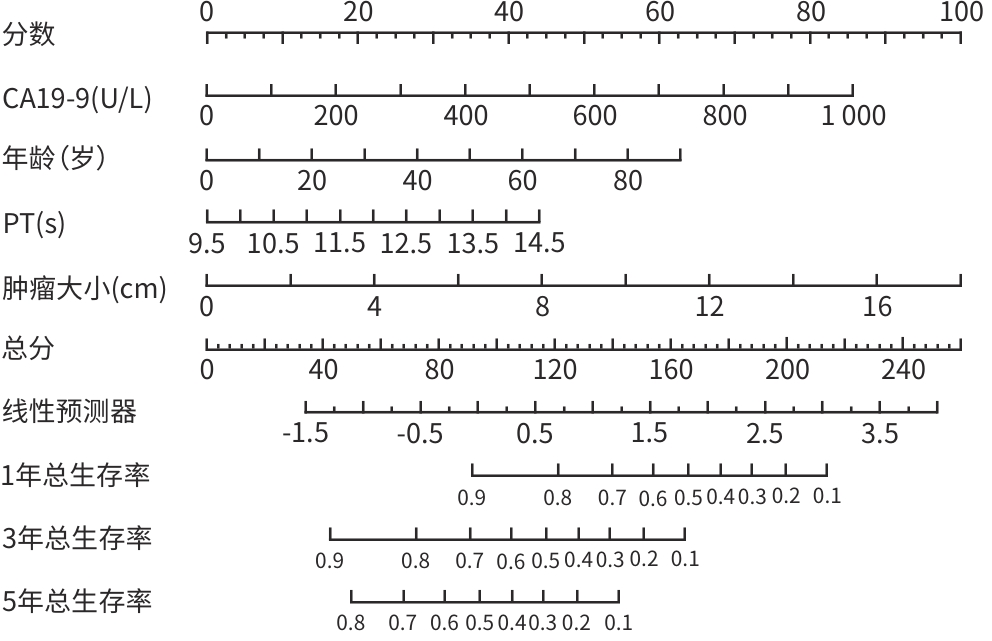
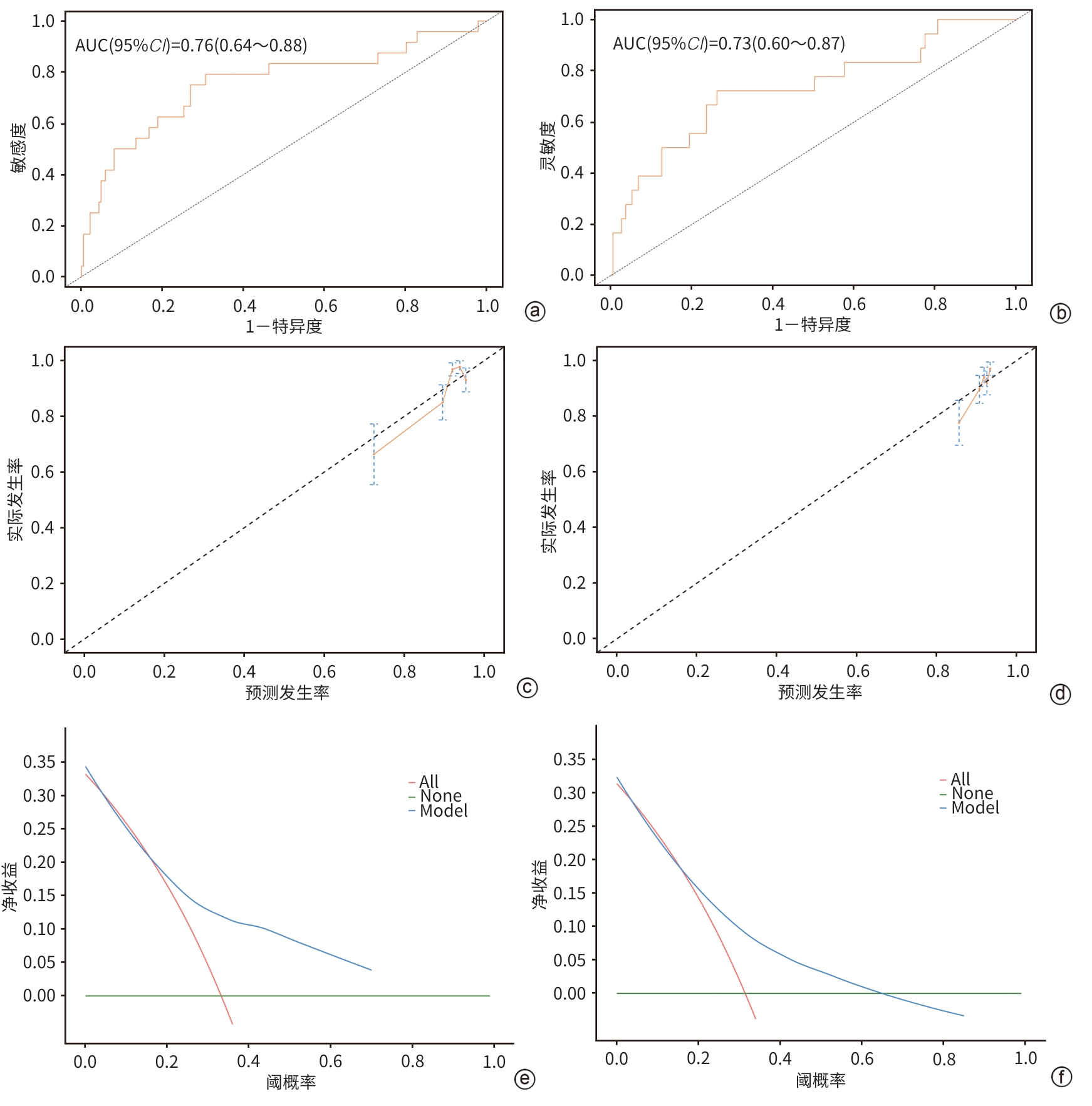
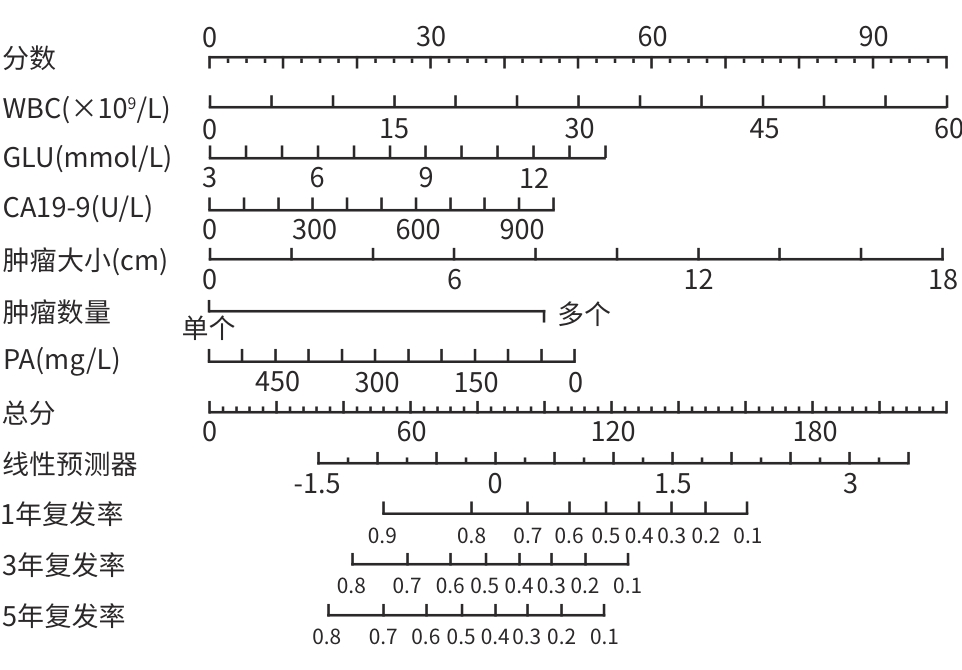
目的 本研究基于多模态临床数据构建AFP阴性肝细胞癌(ANHC)患者术后复发及生存风险的动态列线图模型,通过机器学习整合肿瘤生物学特征与治疗响应参数,揭示ANHC特异性预后标志物组合,为突破传统血清学标志物局限提供个性化风险评估工具。 方法 回顾性纳入2012年4月28日—2018年12月31日于东方肝胆外科医院接受肝切除术的421例ANHC患者,随机分为训练组(210例)和验证组(211例)。通过单因素及多因素Cox比例风险回归筛选独立预后因素,构建列线图模型,并采用受试者操作特征曲线(ROC曲线)、校准曲线和决策曲线分析(DCA)验证性能。检测指标包括前白蛋白(PA)、白细胞(WBC)、肿瘤大小、微血管侵犯等。计数资料两组间比较使用χ2检验或Fisher精确检验,计量资料两组间比较使用成组t检验或Mann-Whitney U检验。 结果 通过多因素分析确定的无病生存期(DFS)的独立危险因素包括多发肿瘤(HR=3.30,P<0.001)、WBC(HR=1.05,P=0.005)、血糖(HR=1.15,P=0.026)、糖类抗原19-9(CA19-9)(HR=1.01,P=0.005)和肿瘤大小(HR=1.17,P<0.001),PA为保护因素(HR=0.99,P=0.022)。总生存期(OS)的独立危险因素包括肿瘤包膜不完整(HR=0.60,P=0.009)、年龄(HR=1.02,P=0.035)、凝血酶原时间(PT)(HR=1.27,P=0.023)、CA19-9(HR=1.01,P<0.001)和肿瘤大小(HR=1.15,P<0.001)。DFS列线图在训练组和验证组ROC曲线下面积(AUC)分别为0.74(95%CI:0.64~0.84)和0.67(95%CI:0.57~0.77),OS列线图的AUC分别为0.76(95%CI:0.64~0.88)和0.73(95%CI:0.60~0.87)。校准曲线和DCA表明模型具有良好的预测性能和临床实用性。 结论 肿瘤数目、PA、WBC、肿瘤大小等术前指标可有效预测ANHC患者术后复发,而肿瘤包膜完整性、年龄、PT等与OS显著相关。构建的列线图模型验证性能良好,可为个体化预后评估提供依据。 Abstract:Objective To establish dynamic nomogram models for postoperative recurrence and survival risk of patients with AFP-negative hepatocellular carcinoma (ANHC) based on multimodal clinical data, to identify ANHC-specific prognostic biomarker combinations by integrating tumor biological characteristics and treatment response parameters through machine learning, and to provide an individualized risk assessment tool for overcoming the limitations of traditional serum biomarkers. Methods A retrospective analysis was performed for 421 ANHC patients who underwent hepatectomy in Eastern Hepatobiliary Surgery Hospital from April 2012 to December 2018, and they were randomly divided into training group with 210 patients and validation group with 211 patients. The univariate and multivariate Cox proportional-hazards regression models were used to identify independent prognostic factors and establish a nomogram model, and the receiver operating characteristic (ROC) curve, the calibration curve, and the decision curve analysis were used to assess the performance of the model. Related indicators were measured, including prealbumin (PA), white blood cell count (WBC), tumor size, and microvascular invasion. The chi-square test or the Fisher’s exact test was used for comparison of categorical variables between two groups, and the independent-samples t test or the Mann-Whitney U test was used for comparison of continuous variables between two groups. Results The multivariate analysis showed that multiple tumors (hazard ratio [HR]=3.30, P<0.001), WBC (HR=1.05, P=0.005), blood glucose (HR=1.15, P=0.026), CA19-9 (HR=1.17, P=0.005), and tumor size (HR=1.17, P<0.001) were independent risk factors for disease-free survival (DFS), while PA (HR=0.99, P=0.022) was a protective factor. Incomplete tumor capsule (HR=0.60, P=0.009), age (HR=1.02, P=0.035), prothrombin time (PT) (HR=1.27, P=0.023), CA19-9 (HR=1.01, P<0.001), and tumor size (HR=1.15, P<0.001) were independent risk factors for overall survival (OS). The DFS nomogram achieved an AUC of 0.74 (95% confidence interval [CI]: 0.64 — 0.84) in the training group and 0.67 (95%CI: 0.57 — 0.77) in the validation group, while the OS nomogram had an AUC of 0.76 (95%CI: 0.64 — 0.88) and 0.73 (95%CI: 0.60 — 0.87), respectively. The calibration curve and the decision curve analysis showed that the models had good predictive accuracy and clinical practicability. Conclusion Preoperative indicators, including tumor number, PA, WBC, and tumor size, can effectively predict postoperative recurrence in ANHC patients, while tumor capsule integrity, age, and PT are significantly associated with OS. The nomogram models established have good performance and can provide a basis for individualized prognostic assessment. -
Key words:
- alpha-Fetoproteins /
- Carcinoma, Hepatocellular /
- Nomogram /
- Prognosis
-
表 1 训练组和验证组患者基线资料比较
Table 1. The comparison of baseline data between the training group and the validation group
指标 训练组(n=210) 验证组(n=211) 统计值 P值 年龄(岁) 53.57±10.60 53.81±11.94 t=0.21 0.831 男/女[例(%)] 178(84.76)/32(15.24) 181(85.78)/30(14.22) χ2=0.09 0.768 糖尿病[例(%)] χ2=0.40 0.528 是/否 26(12.38)/184(87.62) 22(10.43)/189(89.57) 抗病毒治疗[例(%)] χ2=2.49 0.115 是/否 18(8.57)/192(91.43) 10(4.74)/201(95.26) 吸烟[例(%)] χ2=0.00 0.970 是/否 78(37.14)/132(62.86) 78(36.97)/133(63.03) 饮酒[例(%)] χ2=0.52 0.470 是/否 57(27.14)/153(72.86) 64(30.33)/147(69.67) 肝癌家族史[例(%)] χ2=0.00 0.999 是/否 3(1.43)/207(98.57) 4(1.90)/207(98.10) 肝硬化[例(%)] χ2=1.60 0.206 是/否 136(64.76)/74(35.24) 124(58.77)/87(41.23) WBC (×109/L) 5.26(4.02~6.98) 5.64(4.72~7.01) Z=-2.71 0.007 RBC(×1012/L) 4.51(4.13~4.80) 4.56(4.30~4.86) Z=-1.93 0.054 PLT(×109/L) 154.50(105.75~203.50) 155.00(119.00~194.00) Z=-0.43 0.671 PDW[例(%)] χ2=1.50 0.220 ≥17/<17 95(45.24)/115(54.76) 83(39.34)/128(60.66) TBil(μmol/L) 12.70(9.33~16.70) 12.80(9.65~16.90) Z=-0.29 0.769 DBil(μmol/L) 4.70(3.32~6.40) 4.80(3.55~6.10) Z=-0.01 0.996 Alb(g/L) 41.40(38.73~43.98) 42.40(39.80~44.40) Z=-1.81 0.070 PA(mg/L) 230.00(189.00~271.75) 237.00(194.00~278.50) Z=-1.54 0.124 ALT(U/L) 34.30(24.10~57.00) 37.00(23.45~58.90) Z=-0.45 0.651 Cr(μmol/L) 69.00(61.25~77.00) 70.00(60.50~78.50) Z=-0.83 0.407 GLU(mmol/L) 5.00(4.60~5.68) 5.00(4.50~5.50) Z=-0.54 0.588 PT(s) 11.95(11.40~12.60) 11.80(11.30~12.40) Z=-1.53 0.127 AFP(ng/mL) 3.25(2.20~5.20) 3.40(2.35~5.20) Z=-0.80 0.423 CEA(μg/L) 2.30(1.52~3.40) 2.20(1.50~3.50) Z=-0.72 0.474 CA19-9(U/L) 16.85(9.60~30.40) 20.00(11.30~36.05) Z=-1.49 0.137 HBsAg[例(%)] χ2=0.10 0.751 阳性/阴性 162(77.14)/48(22.86) 160(75.83)/51(24.17) 抗-HBs[例(%)] χ2=0.37 0.544 阳性/阴性 36(17.14)/174(82.86) 41(19.43)/170(80.57) HBeAg[例(%)] χ2=0.14 0.712 阳性/阴性 52(24.76)/158(75.24) 49(23.22)/162(76.78) 抗-HBe[例(%)] χ2=0.61 0.433 阳性/阴性 141(67.14)/69(32.86) 134(63.51)/77(36.49) 抗-HBc[例(%)] χ2=0.67 0.412 阳性/阴性 186(88.57)/24(11.43) 192(91.00)/19(9.00) 肿瘤大小(cm) 5.39±3.72 5.14±3.07 t=0.76 0.449 肿瘤数量[例(%)] χ2=0.19 0.665 单个/多个 13(6.19)/197(93.81) 11(5.21)/200(94.79) 肿瘤包膜完整[例(%)] χ2=5.70 0.017 是/否 156(74.29)/54(25.71) 134(63.51)/77(36.49) MVI存在[例(%)] χ2=0.86 0.355 是/否 101(48.10)/109(51.90) 92(43.60)/119(56.40) 表 2 训练组DFS的单变量和多变量Cox回归分析
Table 2. Univariate and multivariate Cox regression analysis of DFS in the training group
指标 HR(95%CI) P值 HR(95%CI) P值 年龄 1.00(0.98~1.01) 0.566 性别(男) 1.20(0.69~2.10) 0.519 糖尿病(是) 1.31(0.76~2.25) 0.332 抗病毒治疗(是) 1.89(0.88~4.07) 0.102 吸烟(是) 0.93(0.64~1.35) 0.694 饮酒(是) 0.91(0.62~1.36) 0.657 肝癌家族史(是) 1.74(0.55~5.49) 0.343 肝硬化(是) 1.18(0.81~1.71) 0.395 WBC 1.05(1.02~1.08) <0.001 1.05(1.01~1.09) 0.005 RBC 0.86(0.60~1.22) 0.386 PLT 1.00(0.98~1.02) 0.709 PDW(≥17) 1.20(0.83~1.73) 0.330 TBil 1.01(0.99~1.03) 0.229 DBil 1.03(0.98~1.08) 0.265 Alb 1.01(0.98~1.04) 0.407 PA 0.99(0.98~1.00) <0.001 0.99(0.98~1.00) 0.022 ALT 1.00(0.98~1.01) 0.414 Cr 1.00(0.99~1.01) 0.954 GLU 1.09(0.96~1.23) 0.069 1.15(1.02~1.30) 0.026 PT 1.11(0.91~1.36) 0.288 CEA 1.01(0.98~1.02) <0.001 CA19-9 1.01(1.00~1.02) 0.002 1.01(1.00~1.02) 0.005 HBsAg(阳性) 0.95(0.62~1.46) 0.807 抗-HBs(阳性) 0.87(0.54~1.41) 0.568 HBeAg(阳性) 1.25(0.83~1.88) 0.277 抗-HBe(阳性) 0.83(0.57~1.20) 0.314 抗-HBc(阳性) 0.93(0.48~1.77) 0.815 肿瘤大小 1.17(1.10~1.24) <0.001 1.17(1.10~1.24) <0.001 肿瘤数目(多个) 3.13(1.67~5.85) <0.001 3.30(1.71~6.39) <0.001 肿瘤包膜(不完整) 0.76(0.52~1.11) 0.158 MVI(存在) 1.20(0.83~1.73) 0.326 表 3 训练组OS的单变量和多变量Cox回归分析
Table 3. Univariate and multivariate Cox regression analysis of OS in the training group
指标 HR(95%CI) P值 HR(95%CI) P值 年龄 1.01(1.00~1.03) 0.090 1.02(1.01~1.04) 0.035 性别(男) 0.99(0.57~1.70) 0.968 糖尿病(是) 0.65(0.33~1.29) 0.221 抗病毒治疗(是) 1.10(0.45~2.71) 0.830 吸烟(是) 0.89(0.61~1.31) 0.561 饮酒(是) 0.79(0.52~1.20) 0.270 肝癌家族史(存在) 0.90(0.22~3.67) 0.888 肝硬化(是) 0.86(0.59~1.25) 0.427 WBC 1.01(0.98~1.05) 0.480 RBC 0.80(0.56~1.14) 0.219 PLT 1.00(0.99~1.01) 0.319 PDW(≥17) 0.97(0.66~1.42) 0.859 TBil 1.01(0.99~1.03) 0.416 DBil 1.01(0.95~1.07) 0.726 Alb 0.99(0.97~1.02) 0.687 PA 0.99(0.98~1.00) <0.001 ALT 1.00(0.99~1.01) 0.894 Cr 0.99(0.97~1.00) 0.051 GLU 1.01(0.88~1.16) 0.870 PT 1.19(0.97~1.46) 0.094 1.27(1.03~1.55) 0.023 CEA 1.01(1.00~1.02) <0.001 CA19-9 1.01(1.00~1.02) <0.001 1.01(1.00~1.02) <0.001 HBsAg(阳性) 0.65(0.43~0.98) 0.039 抗-HBs(阳性) 1.24(0.79~1.94) 0.354 HBeAg(阳性) 0.89(0.56~1.39) 0.596 抗-HBe(阳性) 0.83(0.57~1.21) 0.334 抗-HBc(阳性) 0.78(0.43~1.43) 0.427 肿瘤大小 1.18(1.11~1.25) <0.001 1.15(1.08~1.22) <0.001 肿瘤数目(多个) 1.29(0.60~2.78) 0.513 肿瘤包膜(不完整) 0.53(0.37~0.77) <0.001 0.60(0.40~0.88) 0.009 MVI(存在) 1.11(0.77~1.62) 0.574 -
[1] SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2022[J]. CA A Cancer J Clin, 2022, 72( 1): 7- 33. DOI: 10.3322/caac.21708. [2] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380( 15): 1450- 1462. DOI: 10.1056/nejmra1713263. [3] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16( 10): 589- 604. DOI: 10.1038/s41575-019-0186-y. [4] YANG SL, LIU LP, YANG S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma[J]. Br J Surg, 2016, 103( 6): 716- 724. DOI: 10.1002/bjs.10093. [5] SUN LY, CEN WJ, ZENG XX, et al. The prognostic value of alpha-fetoprotein ratio in patients with resectable alpha-fetoprotein-negative hepatocellular carcinoma[J]. Am Surg, 2024, 90( 6): 1240- 1249. DOI: 10.1177/00031348241227202. [6] LIN KY, CHEN QJ, GUO LB, et al. The evaluation of alpha-fetoprotein response on efficacy and prognosis in targeted therapy combined with immunotherapy for intermediate-to-advanced hepatocellular carcinoma: a multicenter clinical study[J]. Chin J Dig Surg, 2024, 23( 2): 248- 256. DOI: 10.3760/cma.j.cn115610-20231128-00219.林孔英, 陈清静, 郭洛彬, 等. 甲胎蛋白反应评估中晚期肝癌靶免联合治疗效果和预后的多中心临床研究[J]. 中华消化外科杂志, 2024, 23( 2): 248- 256. DOI: 10.3760/cma.j.cn115610-20231128-00219. [7] HAN LL, LV Y, GUO H, et al. Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy[J]. World J Gastroenterol, 2014, 20( 30): 10249- 10261. DOI: 10.3748/wjg.v20.i30.10249. [8] CHEN SS, CHEN H, GAO SS, et al. Differential expression of plasma microRNA-125b in hepatitis B virus-related liver diseases and diagnostic potential for hepatitis B virus-induced hepatocellular carcinoma[J]. Hepatol Res, 2017, 47( 4): 312- 320. DOI: 10.1111/hepr.12739. [9] JING W, PENG RY, ZHU M, et al. Differential expression and diagnostic significance of pre-albumin, fibrinogen combined with D-dimer in AFP-negative hepatocellular carcinoma[J]. Pathol Oncol Res, 2020, 26( 3): 1669- 1676. DOI: 10.1007/s12253-019-00752-8. [10] YANG CK, WU XY, LIU JY, et al. Nomogram based on platelet-albumin-bilirubin for predicting tumor recurrence after surgery in alpha-fetoprotein-negative hepatocellular carcinoma patients[J]. J Hepatocell Carcinoma, 2023, 10: 43- 55. DOI: 10.2147/JHC.S396433. [11] LIN KY, HUANG QZ, ZENG JX, et al. Clinical significance of alpha-fetoprotein in alpha-fetoprotein negative hepatocellular carcinoma underwent curative resection[J]. Dig Dis Sci, 2021, 66( 12): 4545- 4556. DOI: 10.1007/s10620-020-06797-z. [12] HUANG J, LIU FC, LI L, et al. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection[J]. Cancer Med, 2020, 9( 8): 2791- 2802. DOI: 10.1002/cam4.2944. [13] WANG LY, FENG B, LIANG M, et al. Prognostic performance of MRI LI-RADS version 2018 features and clinical-pathological factors in alpha-fetoprotein-negative hepatocellular carcinoma[J]. Abdom Radiol(NY), 2024, 49( 6): 1918- 1928. DOI: 10.1007/s00261-024-04278-9. [14] ZHANG ZG, ZHANG YY, WANG YY, et al. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma[J]. Onco Targets Ther, 2015, 9: 123- 129. DOI: 10.2147/OTT.S90732. [15] WANG T, LIU M, ZHENG SJ, et al. Tumor-associated autoantibodies are useful biomarkers in immunodiagnosis of α-fetoprotein-negative hepatocellular carcinoma[J]. World J Gastroenterol, 2017, 23( 19): 3496- 3504. DOI: 10.3748/wjg.v23.i19.3496. [16] LI YZ, WANG HY, REN DF, et al. Interleukin-41: A novel serum marker for the diagnosis of alpha-fetoprotein-negative hepatocellular carcinoma[J]. Front Oncol, 2024, 14: 1408584. DOI: 10.3389/fonc.2024.1408584. [17] LIU Y, LI T, ZHANG HL, et al. Establishment and validation of a gene mutation-based risk model for predicting prognosis and therapy response in acute myeloid leukemia[J]. Heliyon, 2024, 10( 10): e31249. DOI: 10.1016/j.heliyon.2024.e31249. [18] YANG YY, TANG P, YU ZT, et al. Value of number of negative lymph nodes in predicting the prognosis of patients with esophageal cancer after neoadjuvant therapy and the construction of nomogram prodiction model[J]. Chin J Dig Surg, 2023, 22( 3): 371- 382. DOI: 10.3760/cma.j.cn115610-20230216-00066.杨月阳, 唐鹏, 于振涛, 等. 阴性淋巴结数目对新辅助治疗食管癌患者预后的预测价值及列线图预测模型构建[J]. 中华消化外科杂志, 2023, 22( 3): 371- 382. DOI: 10.3760/cma.j.cn115610-20230216-00066. [19] ZHANG HH, SHENG SG, QIAO WY, et al. Nomogram built based on machine learning to predict recurrence in early-stage hepatocellular carcinoma patients treated with ablation[J]. Front Oncol, 2024, 14: 1395329. DOI: 10.3389/fonc.2024.1395329. [20] TANG XM, WANG Q, JIN RH, et al. A novel nomogram to predict prognosis in elderly early-stage hepatocellular carcinoma patients after ablation therapy[J]. J Hepatocell Carcinoma, 2024, 11: 901- 911. DOI: 10.2147/JHC.S459250. [21] WANG Q, QIAO WY, ZHANG HH, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma[J]. Front Immunol, 2022, 13: 1019638. DOI: 10.3389/fimmu.2022.1019638. [22] ZHU Y, GU LL, CHEN T, et al. Factors influencing early recurrence of hepatocellular carcinoma after curative resection[J]. J Int Med Res, 2020, 48( 8): 300060520945552. DOI: 10.1177/0300060520945552. [23] LI J, TAO HS, ZHANG EL, et al. Diagnostic value of gamma-glutamyl transpeptidase to alkaline phosphatase ratio combined with gamma-glutamyl transpeptidase to aspartate aminotransferase ratio and alanine aminotransferase to aspartate aminotransferase ratio in alpha-fetoprotein-negative hepatocellular carcinoma[J]. Cancer Med, 2021, 10( 14): 4844- 4854. DOI: 10.1002/cam4.4057. [24] HU J, XU Y, SHEN ZZ, et al. High expressions of vascular endothelial growth factor and platelet-derived endothelial cell growth factor predict poor prognosis in alpha-fetoprotein-negative hepatocellular carcinoma patients after curative resection[J]. J Cancer Res Clin Oncol, 2009, 135( 10): 1359- 1367. DOI: 10.1007/s00432-009-0577-5. [25] LU LH, ZHANG YF, WEI W, et al. Preoperative carbohydrate antigen 19-9: Its neglected role in alpha-fetoprotein-negative hepatocellular carcinoma patients[J]. J Gastrointest Surg, 2017, 21( 12): 2025- 2032. DOI: 10.1007/s11605-017-3528-5. [26] FAN Y, SUN YM, MAN CF, et al. Preoperative serum prealbumin level and adverse prognosis in patients with hepatocellular carcinoma after hepatectomy: A meta-analysis[J]. Front Oncol, 2021, 11: 775425. DOI: 10.3389/fonc.2021.775425. [27] JIA RR, ZHONG JH, HUO RR, et al. Correlation between serum prealbumin and prognosis of patients with hepatocellular carcinoma after hepatectomy[J]. J Surg Oncol, 2019, 119( 6): 794- 800. DOI: 10.1002/jso.25378. [28] ZHANG J, QIN SD, LI Y, et al. Prognostic significance of combined α-fetoprotein and CA19-9 for hepatocellular carcinoma after hepatectomy[J]. World J Surg Oncol, 2022, 20( 1): 346. DOI: 10.1186/s12957-022-02806-9. [29] YU Z, CHEN DM, ZHENG YS, et al. Development and validation of a diagnostic model for AFP-negative hepatocellular carcinoma[J]. J Cancer Res Clin Oncol, 2023, 149( 13): 11295- 11308. DOI: 10.1007/s00432-023-04997-4. [30] SHEN J, ZHOU Y, YU B, et al. Construction and validation of a nomogram for patients with multiple hepatocellular carcinoma: A SEER-based study[J]. Eur J Surg Oncol, 2023, 49( 10): 106966. DOI: 10.1016/j.ejso.2023.06.018. [31] ZHANG BL, LIU J, DIAO GH, et al. Construction and validation of a novel nomogram predicting recurrence in alpha-fetoprotein-negative hepatocellular carcinoma post-surgery using an innovative liver function-nutrition-inflammation-immune(LFNII) score: A bicentric investigation[J]. J Hepatocell Carcinoma, 2024, 11: 489- 508. DOI: 10.2147/JHC.S451357. -



 PDF下载 ( 2028 KB)
PDF下载 ( 2028 KB)

 下载:
下载: