G-GADA模型在HBV相关肝细胞癌诊断中的应用价值
DOI: 10.12449/JCH250819
The application value of G-GADA model in the diagnosis of hepatitis B virus-related hepatocellular carcinoma
-
摘要:
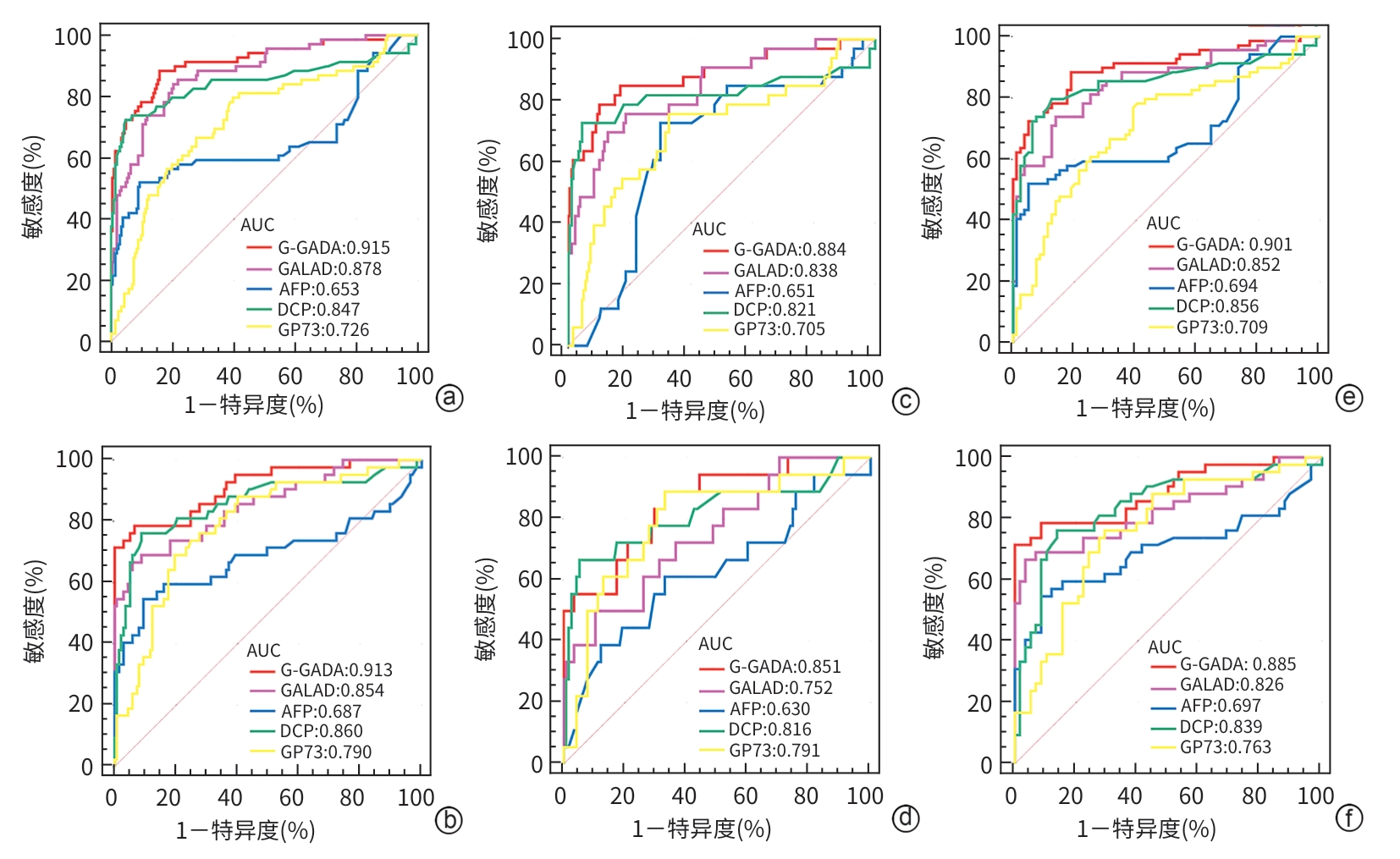
目的 基于慢性乙型肝炎(CHB)患者年龄、性别、甲胎蛋白(AFP)、异常凝血酶原(DCP)和高尔基体蛋白73(GP73),构建优化的肝细胞癌(HCC)诊断模型(G-GADA),以应对HBV相关HCC早期诊断的低敏感度和特异度问题,并评估其对HCC的诊断价值。 方法 回顾性收集2015年6月—2020年6月福建医科大学孟超肝胆医院的CHB患者201例(CHB组)、HBV相关肝硬化患者137例(LC组)及未经治疗的初诊HCC患者111例(HCC组)。比较血清学指标(AFP、DCP、AFP-L3%、GP73)在不同组间的差异,分析其与HCC患者临床和肿瘤特征的关系,并利用Spearman相关分析法评估各指标之间的相关性。通过Logistic回归建立肝癌诊断模型,采用受试者操作特征曲线(ROC曲线)评价各指标对肝癌的诊断效能。 结果 比较CHB、LC和HCC患者的临床特征,结果显示,HCC患者年龄更大,男性比例更高,血清DCP、AFP、GP73和AFP-L3%水平最高,差异均具有统计学意义(P值均<0.05)。在HCC患者中,DCP水平与肿瘤大小及微血管浸润有关;AFP水平与患者年龄、肿瘤大小、肿瘤数量、远处转移及微血管浸润有关;AFP-L3%水平与患者年龄、肿瘤大小、肿瘤数量、远处转移、米兰分期及微血管浸润有关;GP73水平与肿瘤数量、远处转移及微血管浸润有关(P值均<0.05)。患者血清学指标相关性分析显示,AFP与AFP-L3%呈强正相关(r=0.71,P<0.05)、AFP与GP73(r=0.33,P<0.05)、AFP-L3%与GP73(r=0.41,P<0.05)呈中等相关。以患者年龄、性别、DCP、AFP和GP73水平为特征,基于多变量Logistic回归构建HCC诊断模型“G-GADA”,在总患者中,G-GADA模型在建模组和验证组诊断HCC的ROC曲线下面积(AUC)分别为0.915(95%CI:0.875~0.945)和0.913(95%CI:0.862~0.950);在AFP低表达患者中,G-GADA模型在建模组和验证组诊断HCC的AUC分别为0.884(95%CI:0.833~0.924)和0.851(95%CI:0.779~0.907);在肝硬化患者中,G-GADA模型在建模组和验证组诊断HCC的AUC分别为0.901(95%CI:0.841~0.944)和0.885(95%CI:0.806~0.940)。 结论 基于多变量联合构建的G-GADA诊断模型可显著提高肝癌的检出率,在AFP低表达患者、肝硬化患者中均表现出较好的诊断效能,G-GADA模型在HCC的无创诊断中有更好的临床应用价值。 -
关键词:
- 乙型肝炎, 慢性 /
- 癌, 肝细胞 /
- 甲胎蛋白类 /
- 高尔基体基质蛋白质类 /
- 异常凝血酶原
Abstract:Objective To establish an optimized diagnostic model for hepatocellular carcinoma (HCC), designated as G-GADA, in chronic hepatitis B (CHB) patients based on the parameters of age, sex, alpha-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP), and Golgi protein 73 (GP73), to address the problems of low sensitivity and specificity in the early diagnosis of hepatitis B virus (HBV)-related liver cancer, and to assess the value of this model in the diagnosis of HCC. Methods A retrospective analysis was performed for 201 CHB patients (CHB group), 137 patients with HBV-related liver cirrhosis (LC group), and 111 treatment-naïve patients with newly diagnosed HCC (HCC group) who were admitted to Mengchao Hepatobiliary Hospital of Fujian Medical University from June 2015 to June 2020. Serological markers (AFP, DCP, alpha-fetoprotein L3% [AFP-L3%], and GP73) were compared between groups and were analyzed in terms of their differences from the clinical and tumor characteristics of HCC patients, and the Spearman correlation analysis was used to assess the correlation between different markers. A Logistic regression analysis was used to establish a diagnostic model for liver cancer, and the receiver operating characteristic (ROC) curve was used to assess the diagnostic performance of each marker. Results Comparison of clinical features between CHB, LC, and HCC patients showed that HCC patients had significantly higher age, proportion of male patients, and serum levels of DCP, AFP, GP73, and AFP-L3% (all P<0.05). In HCC patients, DCP levels are associated with tumor size and microvascular invasion; AFP levels are related to patient age, tumor size, tumor number, distant metastasis, and microvascular invasion; AFP-L3% levels are associated with patient age, tumor size, tumor number, distant metastasis, Milan staging, and microvascular invasion; GP73 levels are linked to tumor number, distant metastasis, and microvascular invasion(all P<0.05). The correlation analysis of the serum markers showed a strong positive correlation between AFP and AFP-L3% (r=0.71,P<0.05) and a moderate positive correlation between AFP and GP73 (r=0.33,P<0.05) and between AFP-L3% and GP73 (r=0.41,P<0.05). Based on the features of age, sex, DCP, AFP, and GP73, the multivariate Logistic regression analysis was used to establish a G-GADA diagnostic model for HCC, and for all patients, the G-GADA model had an area under the ROC curve (AUC) of 0.915 (95% confidence interval [CI]:0.875 — 0.945) in the derivation cohort and 0.913 (95%CI:0.862 — 0.950) in the validation cohort for the diagnosis of HCC. In the AFP-negative patients, the G-GADA model achieved an AUC of 0.884 (95%CI:0.833 — 0.924) in the derivation cohort and 0.851 (95%CI:0.779 — 0.907) in the validation cohort, and in the patients with liver cirrhosis, the G-GADA model achieved an AUC of 0.901 (95%CI:0.841 — 0.944) in the derivation cohort and 0.885 (95%CI:0.806 — 0.940) in the validation cohort. Conclusion The G-GADA diagnostic model based on multiple variables significantly improves the detection rate of HCC, and demonstrates superior diagnostic performance in patients with low AFP expression and those with liver cirrhosis. The G-GADA model has a better clinical application value in the noninvasive diagnosis of HCC. -
表 1 研究人群基线特征分析
Table 1. Baseline characteristics of the study population
变量 CHB组(n=201) LC组(n=137) HCC组(n=111) 统计值 P值 年龄(岁) 41(32~52)1) 53(48~62) 54(44~63) H=85.475 <0.001 性别[例(%)] χ2=20.237 <0.001 男 128(63.70)1) 96(70.07)1) 96(86.48) 女 73(36.30) 41(29.93) 15(13.51) DCP(mAU/mL) 25.00(21.00~33.00)1) 24.00(18.00~31.50)1) 176.50(37.00~4 305.50) H=123.674 <0.001 AFP(ng/mL) 5.29(3.40~9.15)1) 4.50(3.00~8.53)1) 9.95(2.85~352.51) H=11.284 0.004 GP73(ng/mL) 82.20(60.12~120.80)1) 92.58(65.98~137.60)1) 148.35(103.31~187.35) H=52.381 <0.001 AFP-L3% 0.10(0.10~0.47)1) 0.10(0.10~0.10)1) 1.00(0.10~32.11) H=60.961 <0.001 注:与HCC组比较,1)P<0.05。
表 2 不同血清学指标与HCC患者临床和肿瘤特征的关系
Table 2. Correlation of different serological markers with clinical and tumour characteristics in HCC patients
变量 例
数DCP[例(%)] χ2值 P值 AFP[例(%)] χ2值 P值 AFP-L3% 统计值 P值 GP73(ng/mL) 统计值 P值 低表达
(n=28)高表达
(n=83)低表达
(n=51)高表达
(n=60)年龄 0.083 0.773 6.706 0.010 Z=-2.556 0.011 Z=-0.420 0.674 ≤50岁 45 12(42.9) 33(39.8) 14(27.5) 31(51.7) 13.25(0.35~132.47) 164.10(113.59~193.03) >50岁 66 16(57.1) 50(60.2) 37(72.5) 29(48.3) 1.00(0.10~20.71) 151.66(103.62~194.02) 性别 0.604 0.437 1.111 0.292 Z=-1.420 0.156 Z=-0.759 0.448 男 96 23(82.1) 73(88.0) 5(9.8) 10(16.7) 24.93(0.10~172.65) 170.70(120.60~197.80) 女 15 5(17.9) 10(12.0) 46(90.2) 50(83.3) 1.88(0.10~32.16) 151.66(103.97~191.57) 肿瘤大小 11.712 <0.001 6.116 0.013 Z=-4.204 <0.001 Z=-1.131 0.258 ≤5 cm 69 25(89.3) 44(53.0) 38(74.5) 31(51.7) 1.00(0.10~11.25) 151.09(103.58~188.72) >5 cm 42 3(10.7) 39(47.0) 13(25.5) 29(48.3) 31.91(1.00~1 272.50) 160.15(119.19~200.28) 肿瘤数量 5.271 0.072 23.138 <0.001 H=21.101 <0.001 H=19.049 <0.001 单个 67 18(64.3) 49(59.0) 43(84.3) 24(40.0) 1.00(0.10~9.00) 132.20(82.68~172.15) 多个 23 2(7.1) 21(25.3) 3(5.9) 20(33.3) 63.75(8.42~4 479.36) 170.70(132.70~236.30) 未知 21 8(28.6) 13(15.7) 5(9.8) 16(26.7) 5.75(0.35~48.60) 194.00(162.50~221.25) 远处转移 3.914 0.141 28.721 <0.001 H=31.292 <0.001 H=14.541 <0.001 否 66 17(60.7) 49(59.0) 44(86.3) 22(36.7) 0.10(0.10~5.63) 138.10(82.56~177.87) 是 24 3(10.7) 21(25.3) 5(9.8) 19(31.7) 302.32(5.56~3 837.02) 160.15(134.20~207.25) 未知 21 8(28.6) 13(15.7) 2(3.9) 19(31.7) 20.36(5.53~54.18) 194.00(151.80~228.90) 微血管浸润 29.795 <0.001 23.505 <0.001 H=22.636 <0.001 H=14.339 <0.001 否 65 17(60.7) 48(57.8) 42(82.4) 23(38.3) 0.60(0.10~6.26) 142.20(99.32~176.58) 是 38 3(10.7) 35(42.2) 9(17.6) 29(48.3) 39.80(0.60~870.00) 169.52(125.18~227.38) 未知 8 8(28.6) 0(0.0) 0(0.0) 8(13.3) 28.79(5.61~72.77) 207.50(173.53~238.03) 米兰分期 0.072 0.789 2.556 0.110 Z=-3.425 <0.001 Z=-1.664 0.096 早期 65 17(60.7) 48(57.8) 34(66.7 31(51.7) 1.00(0.10~17.85) 150.60(103.45~185.59) 晚期 46 11(39.3) 35(42.2) 17(33.3) 29(48.3) 13.37(0.78~1 081.31) 166.09(118.58~216.88) 表 3 建模组和验证组的基线特征比较分析
Table 3. Comparative analysis of baseline characteristics of the model and validation groups
变量 建模组(n=270) 验证组(n=179) P值 CHB(n=122) LC(n=79) HCC(n=69) CHB(n=79) LC(n=58) HCC(n=42) 年龄(岁) 39.50
(30.00~51.00)56.00
(48.00~63.00)52.00
(41.00~63.00)43.00
(34.00~55.00)51.00
(44.00~59.50)56.00
(47.00~63.00)0.18 性别[例(%)] 0.73 男 80(65.60) 52(65.80) 60(87.00) 48(60.80) 44(77.20) 38(88.40) 女 42(34.40) 27(34.20) 9(13.00) 31(39.20) 13(22.80) 5(11.60) DCP(mAU/mL) 25.65
(21.00~33.44)23.00
(18.00~30.00)128.00
(36.50~11 072.50)25.00
(21.00~33.00)25.00
(18.50~36.50)208.00
(37.00~2 300.00)0.76 AFP(ng/mL) 5.15
(3.40~8.95)4.50
(2.50~7.40)33.25
(3.00~368.76)5.29
(3.25~11.10)4.60
(3.22~15.90)5.00
(2.20~91.00)0.85 GP73(ng/mL) 85.47
(60.75~121.55)90.66
(63.79~131.50)152.23
(102.32~193.38)79.64
(55.60~115.30)96.05
(66.75~141.10)144.19
(103.65~184.08)0.79 AFP-L3% 0.10
(0.10~0.50)0.10
(0.10~0.10)2.76
(0.10~59.62)0.10
(0.10~0.25)0.10
(0.10~0.22)0.10
(0.10~16.00)0.48 Alb(g/L) 41.00
(38.00~45.00)39.00
(34.00~43.00)37.00
(34.50~41.00)41.00
(38.00~45.00)39.00
(32.00~43.00)37.00
(32.00~41.00)0.85 PLT(×109/L) 2.63
(2.32~3.02)2.59
(2.12~2.98)2.84
(2.40~3.62)2.68
(2.42~3.10)2.50
(2.14~3.11)3.05
(2.39~3.58)0.74 TBil(μmol/L) 16.50
(12.40~22.85)17.80
(13.00~24.90)24.40
(15.05~38.60)16.80
(12.80~25.00)17.80
(12.40~26.20)20.00
(12.20~48.00)0.57 DBil(μmol/L) 4.00
(2.50~8.45)4.60
(3.00~8.30)8.60
(5.20~16.70)4.30
(2.70~6.70)5.20
(2.70~9.80)5.70
(3.80~15.30)0.44 ALP(U/L) 93.00
(70.00~114.50)88.00
(76.00~131.00)119.00
(83.00~168.50)90.00
(70.00~121.00)96.00
(79.00~135.00)106.00
(77.00~167.00)0.72 ALT(U/L) 174.00
(64.50~404.50)35.00
(21.00~61.00)42.00
(27.50~110.00)160.00
(62.00~429.00)34.00
(25.00~48.00)41.00
(23.00~58.00)0.53 AST(U/L) 92.00
(38.00~190.50)36.00
(24.00~65.00)48.00
(29.50~147.50)103.00
(40.50~211.50)32.00
(26.00~45.00)47.00
(28.00~102.00)0.78 GGT(U/L) 73.00
(32.00~145.00)45.00
(25.00~102.00)101.00
(40.00~206.00)61.00
(32.50~114.00)47.00
(29.00~99.00)60.00
(25.00~175.00)0.21 APRI 1.44
(1.00~2.01)2.67
(1.79~3.45)2.63
(1.79~3.47)1.58
(1.17~2.40)2.50
(1.85~3.33)2.72
(2.12~4.77)0.33 FIB-4 1.61
(0.95~2.57)5.19
(3.15~9.05)4.35
(2.58~6.65)2.09
(1.36~3.44)4.63
(3.03~7.21)6.02
(3.17~10.81)0.19 注:APRI,AST与血小板比值指数;FIB-4,肝纤维化4因子指数。
表 4 患者年龄、性别及血清学指标影响HCC发生的多因素Logistic回归分析
Table 4. Multifactorial Logistic regression analysis of patient age, sex and serological indicators on the occurrence of HCC
变量 B值 SE Waldχ2 P值 OR 95%CI 年龄 0.050 0.017 9.163 0.002 1.051 1.018~1.086 性别 1.778 0.627 8.037 0.005 5.920 1.731~20.240 log10GP73 2.028 0.980 4.287 0.038 7.600 1.114~51.829 log10AFP 1.118 0.309 13.122 <0.001 3.059 1.671~5.602 log10DCP 2.657 0.569 21.841 <0.001 14.259 4.678~43.463 常量 -14.784 2.592 32.537 <0.001 表 5 总患者G-GADA、GALAD、AFP、DCP、GP73对HCC的诊断价值
Table 5. Diagnostic value of G-GADA, GALAD, AFP, DCP, GP73 for HCC in overall patients
项目 AUC(95%CI) cut-off 敏感度
(%)特异度
(%)阳性预测值
(%)阴性预测值
(%)Youden指数 P值 建模组(n=201/69) G-GADA 0.915(0.875~0.945) 0.21 88.41 84.08 65.90 95.22 0.725 <0.000 1 GALAD 0.878(0.832~0.914) 0.45 84.06 79.60 58.43 93.36 0.637 <0.000 1 AFP 0.653(0.593~0.710) 28.90 52.17 90.55 64.63 84.22 0.427 0.000 9 DCP 0.847(0.799~0.888) 48.45 72.46 95.02 83.50 90.62 0.675 <0.000 1 GP73 0.726(0.669~0.778) 96.64 79.71 60.20 40.97 89.05 0.399 <0.000 1 验证组(n=137/42) G-GADA 0.913(0.862~0.950) -0.09 78.57 93.43 77.87 93.05 0.721 <0.000 1 GALAD 0.854(0.793~0.902) 1.93 66.67 94.16 77.65 89.75 0.608 <0.000 1 AFP 0.687(0.613~0.754) 40.49 54.76 90.51 63.04 86.10 0.453 <0.000 1 DCP 0.860(0.801~0.907) 45.00 76.19 91.24 72.73 92.31 0.674 <0.000 1 GP73 0.790(0.722~0.847) 137.40 69.05 80.29 52.14 89.10 0.493 <0.000 1 注:n=样本总数/阳性样本。
表 6 AFP低表达患者G-GADA、GALAD、AFP、DCP、GP73对HCC的诊断价值
Table 6. Diagnostic value of G-GADA, GALAD, AFP, DCP and GP73 for HCC in AFP-low expression patients
项目 AUC(95%CI) cut-off 敏感度
(%)特异度
(%)阳性预测值
(%)阴性预测值
(%)Youden指数 P值 建模组(n=176/33) G-GADA 0.884(0.833~0.924) 0.21 78.79 89.77 57.46 95.50 0.686 <0.000 1 GALAD 0.838(0.781~0.885) -0.14 75.76 81.25 42.92 94.45 0.570 <0.000 1 AFP 0.651(0.582~0.715) 3.25 72.73 69.89 30.67 92.83 0.426 0.003 4 DCP 0.821(0.762~0.870) 48.45 72.73 95.45 73.28 94.68 0.682 <0.000 1 GP73 0.705(0.639~0.766) 102.00 75.76 67.05 30.21 93.36 0.428 0.000 2 验证组(n=115/18) G-GADA 0.851(0.779~0.907) -2.18 88.89 66.96 29.64 97.13 0.559 <0.000 1 GALAD 0.752(0.669~0.822) 0.92 50.00 89.57 42.53 91.62 0.396 0.000 1 AFP 0.630(0.542~0.712) 3.30 61.11 66.96 22.60 91.22 0.281 0.106 8 DCP 0.816(0.739~0.878) 48.00 66.67 66.67 36.70 86.66 0.333 <0.000 1 GP73 0.791(0.712~0.856) 137.40 69.05 80.29 35.96 94.07 0.493 <0.000 1 注:n=样本总数/阳性样本。
表 7 肝硬化患者G-GADA、GALAD、AFP、DCP、GP73对HCC的诊断价值
Table 7. Diagnostic value of G-GADA, GALAD, AFP, DCP and GP73 for HCC in cirrhotic patients
项目 AUC(95%CI) cut-off 敏感度
(%)特异度
(%)阳性预测值
(%)阴性预测值
(%)Youden指数 P值 建模组(n=79/69) G-GADA 0.901(0.841~0.944) 0.21 88.41 81.01 80.42 88.39 0.694 <0.000 1 GALAD 0.852(0.785~0.905) 0.31 86.84 78.11 77.61 86.27 0.650 <0.000 1 AFP 0.694(0.613~0.767) 27.10 52.17 94.94 88.49 68.83 0.471 <0.000 1 DCP 0.856(0.789~0.908) 33.00 79.71 87.34 84.35 82.37 0.671 <0.000 1 GP73 0.709(0.629~0.781) 100.20 78.26 59.49 62.78 75.15 0.378 <0.000 1 验证组(n=58/42) G-GADA 0.885(0.806~0.940) 0.46 71.43 100.00 100.00 82.64 0.714 <0.000 1 GALAD 0.826(0.737~0.894) 1.93 66.67 96.55 92.28 79.59 0.632 <0.000 1 AFP 0.697(0.597~0.785) 36.22 54.76 91.38 81.29 73.20 0.461 0.001 DCP 0.839(0.752~0.905) 45.00 76.19 86.21 79.72 83.19 0.624 <0.000 1 GP73 0.763(0.668~0.842) 125.00 76.19 70.69 64.72 80.11 0.469 <0.000 1 注:n=样本总数/阳性样本。
-
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] WANG YX, PAN KF, LI WQ. Interpretation on the report of global cancer statistics 2022[J/CD]. J Multidiscip Cancer Manag Electron Version, 2024, 10( 3): 1- 16.王裕新, 潘凯枫, 李文庆. 2022全球癌症统计报告解读[J/CD]. 肿瘤综合治疗电子杂志, 2024, 10( 3): 1- 16. [3] EL-SERAG HB. Epidemiology of viral hepatitis and hepatocellular carcinoma[J]. Gastroenterology, 2012, 142( 6): 1264- 1273. e 1. DOI: 10.1053/j.gastro.2011.12.061. [4] PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. [5] XIE XF, JIAO L, TIAN B. Value of combined detection of serum miRNA-21, AFP, and AFP-L3 in diagnosis and prognosis evaluation of hepatocellular carcinoma[J]. Chin J Convalescent Med, 2023, 32( 3): 318- 322. DOI: 10.13517/j.cnki.ccm.2023.03.022.解学芳, 焦玲, 田斌. 血清miRNA-21、 AFP、 AFP-L3联合检测在肝细胞癌诊断和预后评估中的价值[J]. 中国疗养医学, 2023, 32( 3): 318- 322. DOI: 10.13517/j.cnki.ccm.2023.03.022. [6] YANG YW, CHEN XL, QU RL, et al. Expression and clinical significance of Th17/Treg cytokines, VEGF, and GP73 in HBV-infected patients[J]. Int Med Health Guid News, 2021, 27( 23): 3639- 3643. DOI: 10.3760/cma.j.issn.1007-1245.2021.23.010.杨勇卫, 陈锡莲, 曲人亮, 等. 慢性HBV感染者Th17/Treg细胞因子、 VEGF、 GP73表达水平差异与临床意义研究[J]. 国际医药卫生导报, 2021, 27( 23): 3639- 3643. DOI: 10.3760/cma.j.issn.1007-1245.2021.23.010. [7] CAVIGLIA GP, RIBALDONE DG, ABATE ML, et al. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: A meta-analysis[J]. Scand J Gastroenterol, 2018, 53( 6): 734- 740. DOI: 10.1080/00365521.2018.1459824. [8] SARTORIUS K, SARTORIUS B, WINKLER C, et al. The biological and diagnostic role of miRNA’s in hepatocellular carcinoma[J]. Front Biosci(Landmark Ed), 2018, 23( 9): 1701- 1720. DOI: 10.2741/4668. [9] LI CX, LI RT, ZHANG W. Progress in non-invasive detection of liver fibrosis[J]. Cancer Biol Med, 2018, 15( 2): 124- 136. DOI: 10.20892/j.issn.2095-3941.2018.0018. [10] WU XY, LI KC, LI CJ, et al. The diagnostic value of GALAD model in Chinese population with hepatocellular carcinoma[J]. Chin J Health Lab Technol, 2022, 32( 15): 1871- 1874.吴小娅, 李克诚, 李纯建, 等. GALAD模型在中国肝癌人群中的诊断价值研究[J]. 中国卫生检验杂志, 2022, 32( 15): 1871- 1874. [11] LIU MX, WU RH, LIU X, et al. Validation of the GALAD model and establishment of GAAP model for diagnosis of hepatocellular carcinoma in Chinese patients[J]. J Hepatocell Carcinoma, 2020, 7: 219- 232. DOI: 10.2147/JHC.S271790. [12] National Health and Family Planning Commission of the People’s Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma(V2017)[J]. J Clin Hepatol, 2017, 33( 8): 1419- 1431. DOI: 10.3969/j.issn.1001-5256.2017.08.003.中华人民共和国国家卫生和计划生育委员会. 原发性肝癌诊疗规范(2017年版)[J]. 临床肝胆病杂志, 2017, 33( 8): 1419- 1431. DOI: 10.3969/j.issn.1001-5256.2017.08.003. [13] National Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [14] MAZZAFERRO V, REGALIA E, DOCI R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis[J]. N Engl J Med, 1996, 334( 11): 693- 699. DOI: 10.1056/NEJM199603143341104. [15] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [16] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(2022 edition)[J]. Pract J Liver Dis, 2023, 26( 3): 457- 478. DOI: 10.3969/j.issn.1672-5069.2023.03.040.中华医学会肝病学分会, 中华医学会感染病学分会. 尤红, 王福生, 李太生, 等. 慢性乙型肝炎防治指南(2022年版)[J]. 实用肝脏病杂志, 2023, 26( 3): 457- 478. DOI: 10.3969/j.issn.1672-5069.2023.03.040. [17] YANG JD, ADDISSIE BD, MARA KC, et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score[J]. Cancer Epidemiol Biomark Prev, 2019, 28( 3): 531- 538. DOI: 10.1158/1055-9965.epi-18-0281. [18] SONG JY, YUAN Q, CHE YD, et al. Performance analysis of predictive model for diagnosis of hepatocellular carcinoma based on PIVKA-II, AFP detection and machine learning algorithms[J]. Chin J Integr Tradit West Med Liver Dis, 2024, 34( 9): 775- 780. DOI: 10.3969/j.issn.1005-0264.2024.009.002.宋佳悦, 袁权, 车雨东, 等. 基于PIVKA-Ⅱ、AFP检测和机器学习算法的肝癌诊断预测模型性能分析[J]. 中西医结合肝病杂志, 2024, 34( 9): 775- 780. DOI: 10.3969/j.issn.1005-0264.2024.009.002. [19] WANG TT, ZHAO SM, LIU XD, et al. Analysis on the external quality assessment results and comparability of detection systems and methods for tumor markers in Shandong province during 2015 and 2017[J]. Chin J Clin Lab Sci, 2019, 37( 4): 310- 313. DOI: 10.13602/j.cnki.jcls.2019.04.17.王恬恬, 赵胜梅, 刘相东, 等. 2015—2017年山东省肿瘤标志物室间质量评价和检测系统及方法间可比性分析[J]. 临床检验杂志, 2019, 37( 4): 310- 313. DOI: 10.13602/j.cnki.jcls.2019.04.17. [20] QI YY, LIN L. Application value of C-GALAD score based on serum AFP, AFP-L3 and DCP levels in the diagnosis of hepatocellular carcinoma[J]. Chin J Clin Lab Sci, 2021, 39( 12): 915- 919. DOI: 10.13602/j.cnki.jcls.2021.12.07.齐莹莹, 林琳. 基于血清AFP、AFP-L3和DCP水平的C-GALAD评分在肝细胞癌诊断中的应用价值[J]. 临床检验杂志, 2021, 39( 12): 915- 919. DOI: 10.13602/j.cnki.jcls.2021.12.07. [21] BEST J, BILGI H, HEIDER D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma[J]. Z Gastroenterol, 2016, 54( 12): 1296- 1305. DOI: 10.1055/s-0042-119529. [22] FENG WX, ZHOU XZ, HAN ZY, et al. Value of GALAD model in diagnosis of HBV-related hepatocellular carcinoma[J]. Chin Hepatol, 2020, 25( 3): 291- 294. DOI: 10.14000/j.cnki.issn.1008-1704.2020.03.020.冯文杏, 周小舟, 韩志毅, 等. GALAD模型在诊断HBV相关肝细胞癌中的价值探讨[J]. 肝脏, 2020, 25( 3): 291- 294. DOI: 10.14000/j.cnki.issn.1008-1704.2020.03.020. [23] XUE JH, CAO ZY, CHENG YN, et al. Acetylation of alpha-fetoprotein promotes hepatocellular carcinoma progression[J]. Cancer Lett, 2020, 471: 12- 26. DOI: 10.1016/j.canlet.2019.11.043. [24] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [25] XIE DY, ZHU K, REN ZG, et al. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: Updates and insights[J]. Hepatobiliary Surg Nutr, 2023, 12( 2): 216- 228. DOI: 10.21037/hbsn-22-469. [26] TIAN S, CHEN YY, ZHANG YM, et al. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma[J]. J Clin Lab Anal, 2023, 37( 1): e24823. DOI: 10.1002/jcla.24823. [27] ZENG H, HUI Y, ZHANG LZ. Study advances on GP73 and the diagnosis and treatment of liver cancer[J]. China Med Pharm, 2021, 11( 15): 58- 61.曾灏, 翚缨, 张林芝. 高尔基体蛋白73与肝癌诊疗相关研究进展[J]. 中国医药科学, 2021, 11( 15): 58- 61. [28] EISSA M, AWAD S, BARAKAT S, et al. Serum Golgi protein 73 as a sensitive biomarker for early detection of hepatocellular carcinoma among Egyptian patients with hepatitis C virus-related cirrhosis[J]. Med J Armed Forces India, 2021, 77( 3): 331- 336. DOI: 10.1016/j.mjafi.2020.11.013. [29] MAO YL, YANG HY, XU HF, et al. Golgi protein 73(GOLPH2) is a valuable serum marker for hepatocellular carcinoma[J]. Gut, 2010, 59( 12): 1687- 1693. DOI: 10.1136/gut.2010.214916. [30] SUN YL, YANG HY, MAO YL, et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival[J]. J Gastroenterol Hepatol, 2011, 26( 7): 1207- 1212. DOI: 10.1111/j.1440-1746.2011.06733.x. [31] VO TD, MAI SH, LAM HT. Evaluating the GALAD score for detection of hepatocellular carcinoma in patients with cirrhosis[J]. J Clin Gastroenterol, 2024. DOI: 10.1097/MCG.0000000000002097. [32] HUANG CJ, FANG M, XIAO X, et al. Validation of the GALAD model for early diagnosis and monitoring of hepatocellular carcinoma in Chinese multicenter study[J]. Liver Int, 2022, 42( 1): 210- 223. DOI: 10.1111/liv.15082. [33] LIU ZY, ZHOU ZH, HE S. Research advances in the role of blood metabolic markers in the treatment response and prognosis prediction of primary liver cancer[J]. J Clin Hepatol, 2023, 39( 10): 2470- 2475. DOI: 10.3969/j.issn.1001-5256.2023.10.028.刘志英, 周智航, 何松. 血液代谢标志物在原发性肝癌治疗反应及预后预测中的应用[J]. 临床肝胆病杂志, 2023, 39( 10): 2470- 2475. DOI: 10.3969/j.issn.1001-5256.2023.10.028. -



 PDF下载 ( 1743 KB)
PDF下载 ( 1743 KB)


 下载:
下载: