失代偿期乙型/丙型肝炎肝硬化患者合并门静脉血栓的列线图模型建立与验证
DOI: 10.12449/JCH250817
Establishment and validation of a nomogram model for patients with decompensated HBV/HCV cirrhosis comorbid with portal vein thrombosis
-
摘要:
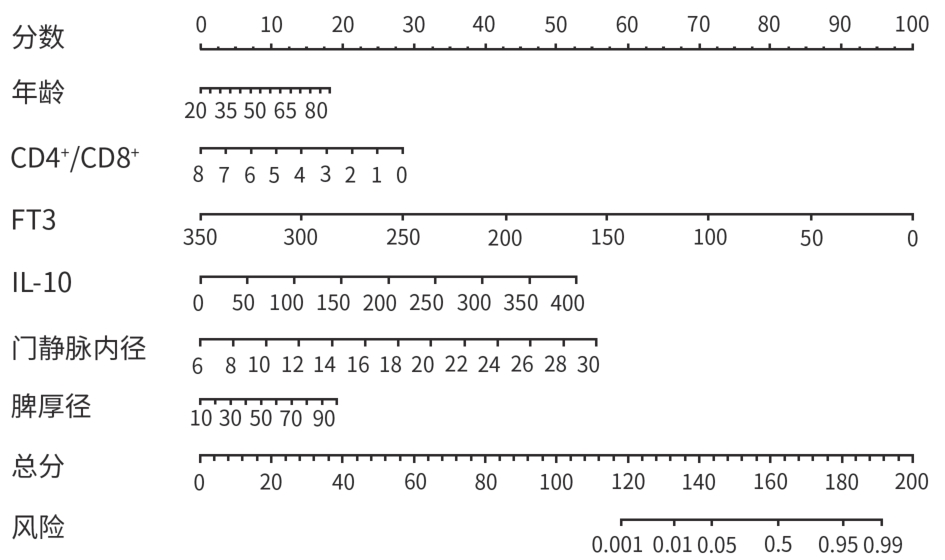
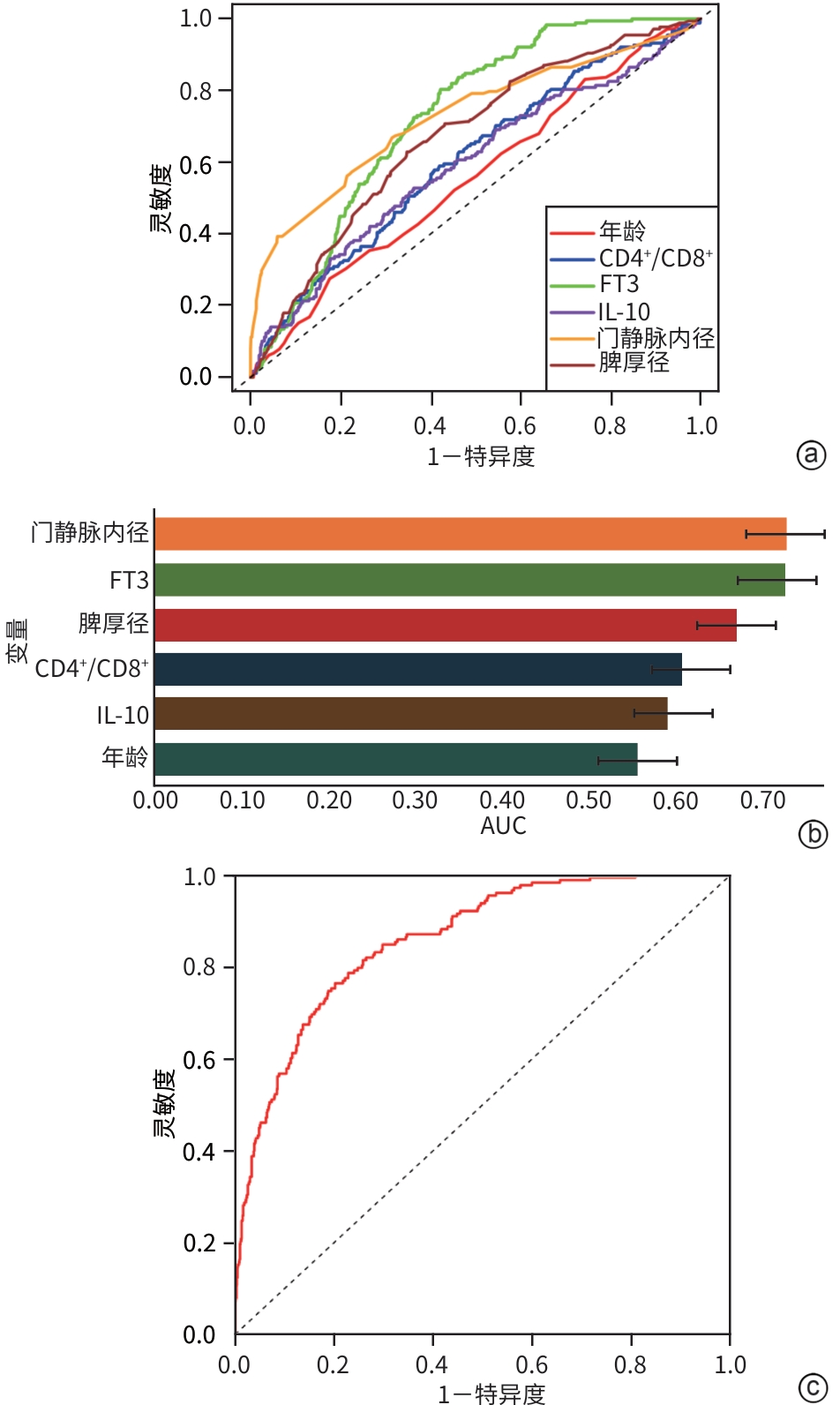
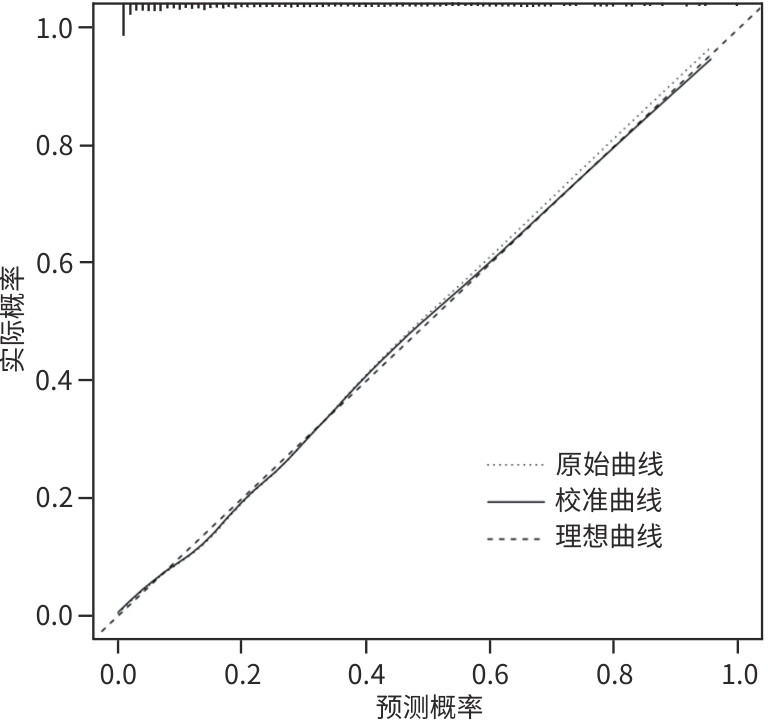
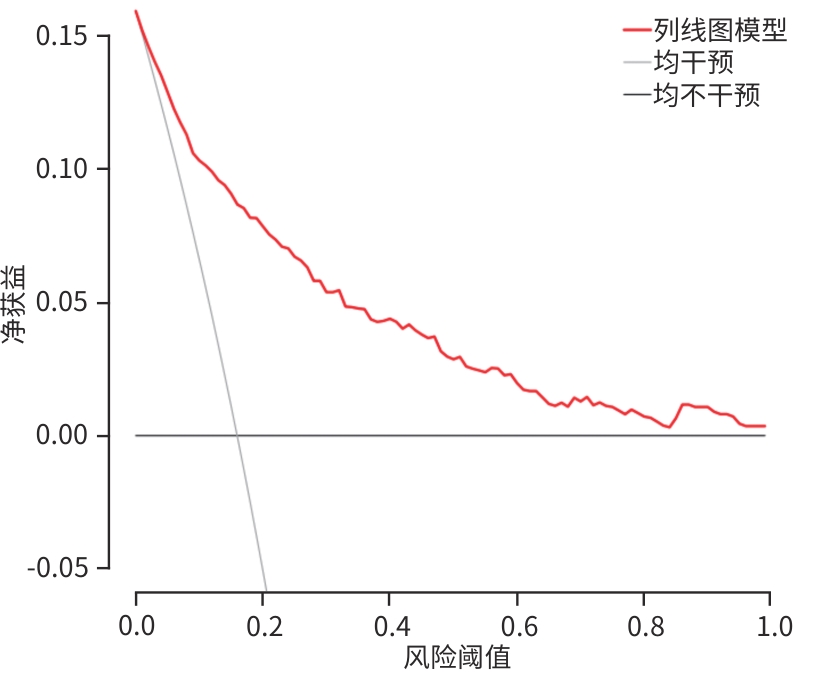
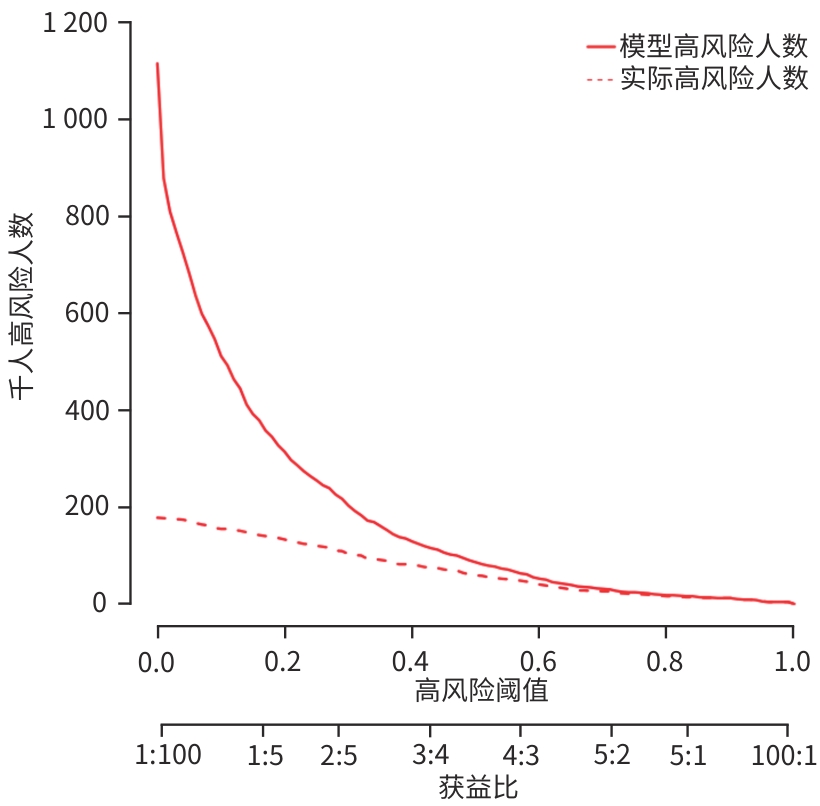
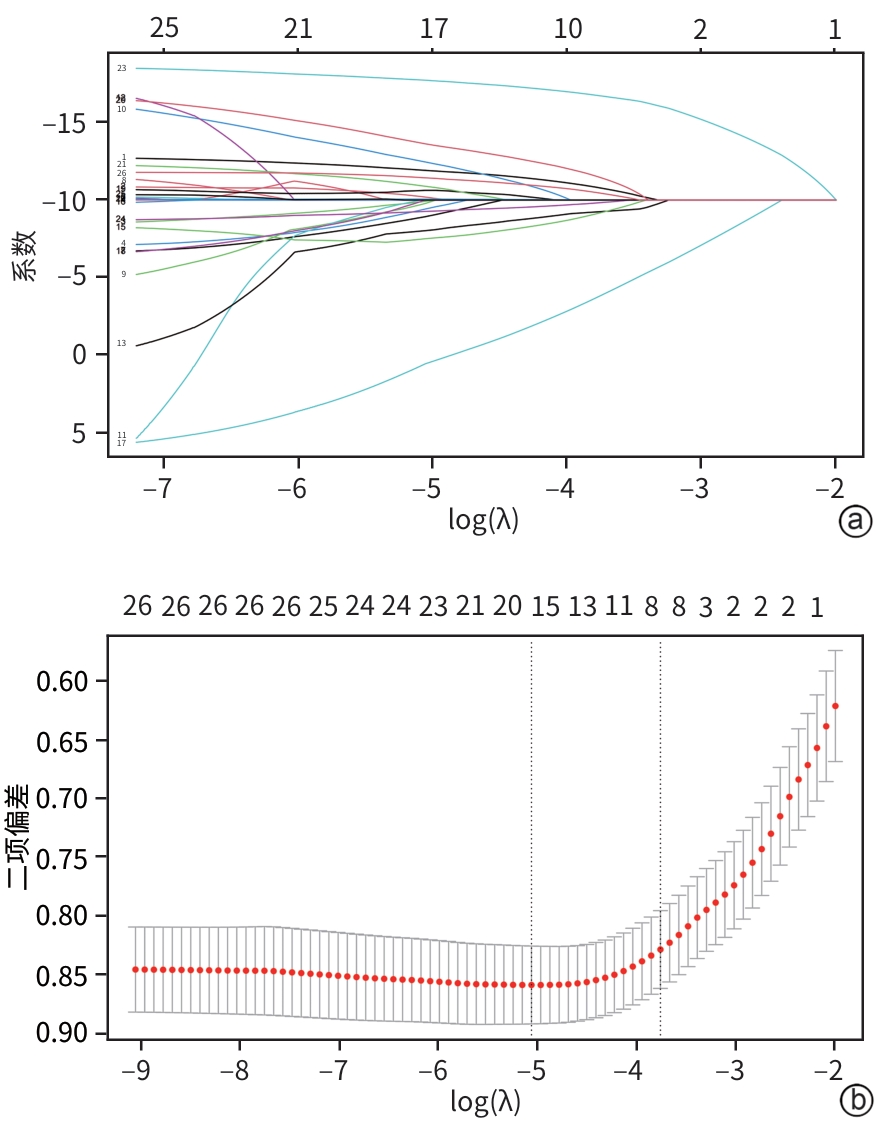
目的 分析失代偿期乙型/丙型肝炎相关肝硬化患者合并门静脉血栓(PVT)的独立影响因素,建立列线图风险预测模型并进行验证。 方法 回顾性收集2022年1月—2023年12月首次在昆明市第三人民医院就诊的1 116例失代偿期乙型/丙型肝炎肝硬化患者的临床资料,并按是否合并PVT分为PVT组和对照组。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。用单因素分析、最小绝对收缩和选择算子(Lasso)回归筛选变量,多因素二元Logistics回归分析筛选独立影响因素并建立预测模型,列线图可视化模型;并使用受试者操作特征曲线(ROC曲线)下面积(AUC)、Hosmer-Lemeshow检验、Bootstrap法自抽样1 000次、校准曲线、临床决策曲线、临床影响曲线验证模型。 结果 PVT组178例,对照组938例,合并PVT患病率15.9%(178/1 116)。男性占68.5%(764/1 116)、饮酒者占51.0%(569/1 116)、Child-Pugh B级者78.8%(879/1 116)、有腹水者67.1%(749/1 116)。PVT组与对照组比较,年龄(Z=-2.362)、凝血酶原时间(Z=-2.403)、国际标准化比值(Z=-2.470)、游离甲状腺素(Z=-5.910)、D-二聚体(Z=-5.764)、IL-6(Z=-6.581)、IL-10(Z=-3.915)、IL-8(Z=-3.705)、门静脉内径(Z=-9.690)、脾厚径(Z=-7.183)升高,而白细胞(Z=-2.115)、血小板(Z=-3.026)、纤维蛋白原(Z=-2.169)、谷氨酸氨基转移酶(Z=-3.151)、前白蛋白(Z=-3.509)、胆碱酯酶(Z=-3.415)、甲胎蛋白(Z=-3.513)、甘油三酯(Z=-2.679)、CD3+(Z=-6.059)、CD4+(Z=-7.257)、CD8+(Z=-2.340)、CD4+/CD8+(Z=-4.479)、三碘甲状腺原氨酸(Z=-3.338)、游离三碘甲状腺原氨酸(FT3)(Z=-9.560)、门静脉血液流速(Z=-4.568)降低,差异均有统计学意义(P值均<0.05)。经Lasso回归筛选出有意义的变量再进行多因素Logistics回归分析,显示年龄(OR=1.046,95%CI:1.026~1.066)、CD4+/CD8+(OR=0.568,95%CI:0.410~0.787)、FT3(OR=0.956,95%CI:0.944~0.968)、IL-10(OR=1.021,95%CI:1.001~1.042)、门静脉内径(OR=1.446,95%CI:1.329~1.574)、脾厚径(OR=1.035,95%CI:1.014~1.055)为合并PVT的独立影响因素,建立模型Logit(P)=-8.784+0.045×年龄-0.566×CD4+/CD8+-0.046×FT3+0.021×IL-10+0.369×门静脉内径+0.034×脾厚径,建立并验证列线图模型,AUC=0.859(95%CI:0.833~0.887)。Hosmer-Lemeshow检验显示,模型拟合较好(χ2=11.349,P=0.183)。Bootstrap内部验证,平均绝对误差=0.006,C指数=0.855。决策曲线显示,在较宽范围内,临床净获益高。 结论 年龄,CD4+/CD8+、FT3、IL-10、门静脉内径、脾厚径可能是失代偿期乙型/丙型肝炎肝硬化患者合并PVT的独立影响因素。以此6个变量建立并验证的预测模型,可能有助于临床上早期预测失代偿期乙型/丙型肝炎肝硬化患者合并PVT的风险。 Abstract:Objective To investigate the independent risk factors for portal vein thrombosis (PVT) in patients with viral hepatitis-related decompensated cirrhosis, and to establish and validate a nomogram risk prediction model. Methods A retrospective analysis was performed for the clinical data of 1 116 patients with decompensated HBV/HCV cirrhosis who attended The Third People’s Hospital of Kunming for the first time from January 2022 to December 2023, and according to the presence or absence of PVT, they were divided into PVT group and control group. The independent samples t-test or the Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. Univariate analysis and least absolute shrinkage and selection operator (LASSO) regression analysis were used to identify variables, and a binary logistic regression analysis was used to obtain independent influencing factors and establish a predictive model, which was visualized using a nomogram. The model was validated based on the receiver operating characteristic (ROC) curve, the area under the ROC curve (AUC), the Hosmer-Lemeshow test, Bootstrap sampling (1 000 iterations), the calibration curve, the decision curve analysis (DCA), and the clinical impact curve (CIC). Results There were 178 patients in the PVT group and 938 patients in the control group, and the prevalence rate of PVT was 15.9%(178/1 116). Male patients accounted for 68.5%(764/1 116), and the patients with drinking, Child-Pugh class B liver function, and ascites accounted for 51.0%(569/1 116),78.8%(879/1 116), and 67.1% (749/1 116), respectively. Compared with the control group, the PVT group had significantly higher age (Z=-2.362,P<0.05), prothrombin time (Z=-2.403,P<0.05), international normalized ratio (Z=-2.470,P<0.05), free thyroxine (Z=-5.910,P<0.05), D-dimer (Z=-5.764,P<0.05), interleukin-6 (Z=-6.581,P<0.05), interleukin-10(IL-10) (Z=-3.915,P<0.05), interleukin-8 (Z=-3.705,P<0.05), diameter of the portal vein (Z=-9.690,P<0.05), and spleen thickness (Z=-7.183,P<0.05), as well as significantly lower levels of white blood cell count (Z=-2.115,P<0.05), platelet count (Z=-3.026,P<0.05), fibrinogen (Z=-2.169,P<0.05), alanine aminotransferase (Z=-3.151,P<0.05), prealbumin (Z=-3.509,P<0.05),cholinesterase (Z=-3.415,P<0.05), alpha-fetoprotein (Z=-3.513,P<0.05), triglycerides (Z=-2.679,P<0.05), CD3 cell count (Z=-6.059,P<0.05), CD4 cell count (Z=-7.257,P<0.05), CD8 cell count (Z=-2.340,P<0.05), CD4+/CD8+ cell ratio (Z=-4.479,P<0.05), triiodothyronine (Z=-3.338,P<0.05), free triiodothyronine (FT3) (Z=-9.560,P<0.05), and portal blood flow velocity (Z=-4.568,P<0.05). The multivariate logistic regression analysis was performed for the variables with statistical significance identified by the LASSO regression analysis, and the results showed that age (odds ratio[OR]=1.046,95% confidence interval[CI]:1.026 — 1.066), CD4+/CD8+ cell ratio (OR=0.568,95%CI:0.410 — 0.787),FT3(OR=0.956,95%CI:0.944 — 0.968), IL-10 (OR=1.021,95%CI:1.001 — 1.042), diameter of the portal vein (OR=1.446,95%CI:1.329 — 1.574), and spleen thickness (OR=1.035,95%CI:1.014 — 1.055) were independent influencing factors. A model was established as Logit(P)=-8.784+0.045×age-0.566×CD4+/CD8+-0.046×FT3+0.021×IL-10+0.369×diameter of the portal vein+0.034×spleen thickness, and a nomogram model was established and validated based on this model, with an AUC of 0.859 (95%CI:0.833 — 0.887). The Hosmer-Lemeshow test showed that the model had a high goodness of fit (χ²=11.349,P=0.183). Bootstrap internal validation showed a mean absolute error of 0.006 and a C-index of 0.855. The decision curve analysis showed that the model had a high net clinical benefit within a wide range of thresholds. Conclusion Age, CD4+/CD8+ ratio, FT3, IL-10, diameter of the portal vein, and spleen thickness may be independent influencing factors for PVT in patients with decompensated HBV/HCV cirrhosis. The predictive model established based on these six variables can help to predict the risk of PVT in patients with hepatitis-related decompensated cirrhosis in the early stage in clinical practice. -
Key words:
- Liver Cirrhosis /
- Thrombosis /
- Nomograms /
- Root Cause Analysis
-
表 1 对照组与PVT一般资料比较
Table 1. Comparison of general information between control group and PVT group
指标 对照组(n=938) PVT组(n=178) 统计值 P值 年龄(岁) 52(45~58) 53(47~60) Z=-2.362 0.018 性别[例(%)] χ2=0.400 0.841 男 641(68) 123(69) 女 297(32) 55(31) 饮酒[例(%)] χ2=0.605 0.437 否 455(49) 92(52) 是 483(51) 86 (48) 吸烟[例(%)] χ2=1.972 0.160 否 405(43) 87(49) 是 533(57) 91(51) 吸毒[例(%)] χ2=0.018 0.894 否 707(75) 135(76) 是 231(25) 43(24) Child-Pugh分级[例(%)] χ2=2.068 0.101 B级 747(80) 132(74) C级 191(20) 46(26) 表 2 对照组与PVT组实验室指标比较
Table 2. Comparison of laboratory testing between control group and PVT group
指标 对照组(n=938) PVT组(n=178) Z值 P值 白细胞(×109/L) 3.79(2.89~5.16) 3.34(2.40~5.35) -2.115 0.034 血小板(×109/L) 76.00(53.25~109.00) 69.00(49.00~89.75) -3.026 0.002 血氨(μmol/L) 34.90(23.00~51.48) 38.15(27.45~52.09) -1.760 0.078 凝血酶原时间(s) 16.20(14.90~17.88) 16.70(15.30~18.17) -2.403 0.016 国际标准化比值 1.35(1.21~1.53) 1.41(1.26~1.55) -2.470 0.014 纤维蛋白原(g/L) 2.06(1.63~2.55) 1.96(1.53~2.36) -2.169 0.030 总胆红素(μmol/L) 23.25(15.10~41.10) 23.45(17.22~40.27) -0.650 0.516 谷氨酸氨基转移酶(U/L) 31.10(23.00~50.00) 28.00(20.00~40.00) -3.151 0.002 门冬氨酸氨基转移酶(U/L) 33.00(21.00~52.00) 31.00(22.00~42.00) -1.523 0.128 总蛋白(g/L) 64.10(57.32~70.30) 62.50(55.10~68.68) -1.842 0.065 白蛋白(g/L) 32.40(27.50~37.60) 31.55(27.52~35.70) -1.880 0.060 前白蛋白(mg/L) 108.05(74.50~155.12) 93.25(65.30~130.10) -3.509 <0.001 γ-谷氨酰转移酶(U/L) 44.00(24.00~90.00) 39.00(22.33~81.97) -1.407 0.159 碱性磷脂酶(U/L) 111.50(84.25~154.00) 110.50(83.25~151.75) -0.354 0.724 胆碱酯酶(U/L) 3 597.50(2 560.75~5 277.50) 3 261.00(2 227.75~4 309.50) -3.415 <0.001 总胆汁酸(μmol/L) 20.70(7.82~47.90) 21.00(9.55~44.13) -0.047 0.963 肌酐(μmol/L) 61.00(50.00~78.00) 64.50(54.00~78.75) -1.353 0.176 尿酸(μmol/L) 327.00(259.00~413.50) 321.50(263.50~391.75) -0.307 0.759 血糖(mmol/L) 5.62(5.01~7.00) 5.94(5.07~7.24) -1.433 0.152 甲胎蛋白(IU/mL) 4.04(2.54~7.23) 3.20(2.06~5.36) -3.513 <0.001 癌胚抗原(ng/mL) 3.02(1.98~4.40) 2.84(2.02~4.09) -0.872 0.384 甘油三酯(mmol/L) 0.81(0.60~1.17) 0.72(0.56~1.04) -2.679 0.007 总胆固醇(mmol/L) 3.21(2.51~3.87) 3.09(2.39~3.82) -1.212 0.226 D-二聚体(nmol/L) 1.68(0.89~3.43) 2.96(1.51~5.29) -5.764 <0.001 三碘甲状腺原氨酸(nmol/L) 1.67(1.30~2.18) 1.45(1.22~1.87) -3.338 <0.001 FT3(pmol/L) 17.17(3.97~72.37) 3.87(3.43~4.94) -9.560 <0.001 游离甲状腺素(pmol/L) 11.55(5.48~14.59) 13.73(11.06~15.26) -5.910 <0.001 甲状腺球蛋白抗体(IU/mL) 15.12(12.94~19.83) 15.41(13.10~27.15) -0.900 0.368 CD3+(个/μL) 682.49(533.05~857.19) 561.98(450.48~717.15) -6.059 <0.001 CD4+(个/μL) 397.01(307.67~516.45) 302.21(238.11~401.43) -7.275 <0.001 CD8+(个/μL) 233.17(170.90~325.12) 214.75(165.37~283.57) -2.340 0.019 CD4+/CD8+ 1.79(1.44~2.19) 1.59(1.26~1.97) -4.479 <0.001 CD19+(个/μL) 178.91(130.20~238.47) 170.90(133.15~238.91) -0.453 0.651 表 3 对照组与PVT组细胞因子比较
Table 3. Comparison of cytokines between control group and PVT group
指标 对照组(n=938) PVT组(n=178) Z值 P值 IL-5(pg/mL) 2.16(1.35~3.60) 2.43(1.36~3.54) -0.620 0.535 干扰素α(pg/mL) 2.26(1.64~3.81) 2.16(1.59~3.57) -0.699 0.503 IL-2(pg/mL) 2.06(1.38~3.60) 1.99(1.37~3.06) -0.522 0.581 IL-6(pg/mL) 8.63(4.28~19.81) 16.13(9.16~32.01) -6.581 <0.001 IL-1β(pg/mL) 4.73(2.04~10.48) 5.43(2.19~10.15) -0.177 0.859 IL-10(pg/mL) 4.19(2.79~6.26) 5.42(3.44~7.90) -3.915 <0.001 IL-γ(pg/mL) 3.70(2.05~6.96) 4.05(1.95~8.26) -0.518 0.605 IL-8(pg/mL) 19.26(7.03~39.57) 28.01(13.00~51.45) -3.705 <0.001 IL-17(pg/mL) 8.53(4.15~14.76) 9.90(4.26~19.64) -1.946 0.052 IL-4(pg/mL) 1.58(1.15~2.24) 1.58(1.16~2.09) -0.435 0.664 IL-12p70(pg/mL) 2.02(1.57~3.10) 2.03(1.48~2.92) -0.530 0.596 抗肿瘤坏死因子α(pg/mL) 2.77(1.67~5.60) 2.83(1.44~5.27) -0.644 0.519 表 4 对照组与PVT组影像学指标比较
Table 4. Comparison of lmaging examination between control group and PVT group
指标 对照组(n=938) PVT组(n=178) 统计值 P值 腹水[例(%)] χ2=24.034 <0.001 无(0 cm) 335(36) 32(18) 少量(<3 cm) 395(42) 86(48) 中量(3~10 cm) 146(16) 41(23) 大量(>10 cm) 62(7) 19(11) 门静脉内径(mm) 11.80(10.00~13.00) 14.00(12.00~16.00) Z=-9.690 <0.001 门静脉血液流速(m/s) 14.90(12.80~17.10) 13.80(12.14~15.67) Z=-4.568 <0.001 脾厚径(mm) 47.00(41.85~53.55) 53.00(47.00~59.00) Z=-7.183 <0.001 肝硬度值(kPa) 20.40(15.31~27.75) 19.74(17.30~29.25) Z=-1.138 0.255 表 5 合并PVT的多因素Logistic回归分析
Table 5. Multivariate Logistic regression analysis of patients with portal vein thrombosis
指标 B值 SE Wald P值 OR 95%CI 年龄 0.045 0.010 4.540 <0.001 1.046 1.026~1.066 CD4+/CD8+ -0.566 0.166 -3.400 <0.001 0.568 0.410~0.787 FT3 -0.046 0.007 -7.050 <0.001 0.956 0.944~0.968 IL-10 0.021 0.010 2.080 0.037 1.021 1.001~1.042 门静脉内径 0.369 0.043 8.550 <0.001 1.446 1.329~1.574 脾厚径 0.034 0.010 3.350 <0.001 1.035 1.014~1.055 -
[1] Hepatobiliary Disease Study Group, Chinese Society of Gastroenterology, Chinese Medical Association. Consensus for management of portal vein thrombosis in liver cirrhosis(2020, Shanghai)[J]. J Clin Hepatol, 2020, 36( 12): 2667- 2674. DOI: 10.3969/j.issn.1001-5256.2020.12.007.中华医学会消化病学分会肝胆疾病学组. 肝硬化门静脉血栓管理专家共识(2020年, 上海)[J]. 临床肝胆病杂志, 2020, 36( 12): 2667- 2674. DOI: 10.3969/j.issn.1001-5256.2020.12.007. [2] WU YR, ZHANG YQ, ZHENG Y, et al. Advances in mechanisms of portal vein thrombosis in liver cirrhosis[J]. Med J Peking Union Med Coll Hosp, 2025, 16( 2): 439- 447. DOI: 10.12290/xhyxzz.2024-0924.武雅荣, 张永强, 郑英, 等. 肝硬化门静脉血栓形成机制研究进展[J]. 协和医学杂志, 2025, 16( 2): 439- 447. DOI: 10.12290/xhyxzz.2024-0924. [3] FACCIA M, AINORA ME, PONZIANI FR, et al. Portal vein thrombosis in cirrhosis: Why a well-known complication is still matter of debate[J]. World J Gastroenterol, 2019, 25( 31): 4437- 4451. DOI: 10.3748/wjg.v25.i31.4437. [4] HUNG HC, LEE JC, CHENG CH, et al. Protein S for portal vein thrombosis in cirrhotic patients waiting for liver transplantation[J]. J Clin Med, 2020, 9( 4): 1181. DOI: 10.3390/jcm9041181. [5] SHALABY S, SIMIONI P, CAMPELLO E, et al. Endothelial damage of the portal vein is associated with heparin-like effect in advanced stages of cirrhosis[J]. Thromb Haemost, 2020, 120( 8): 1173- 1181. DOI: 10.1055/s-0040-1713169. [6] FENG WJ, ZHOU N, WANG YL, et al. Portal vein thrombosis in liver cirrhosis: Risk factors and protection strategies[J]. J Clin Hepatol, 2024, 40( 1): 169- 174. DOI: 10.12449/JCH240128.冯文娟, 周宁, 汪雨露, 等. 肝硬化并发门静脉血栓的危险因素与防护策 略[J]. 临床肝胆病杂志, 2024, 40( 1): 169- 174. DOI: 10.12449/JCH240128. [7] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [8] HARDING DJ, PERERA MT, CHEN F, et al. Portal vein thrombosis in cirrhosis: Controversies and latest developments[J]. World J Gastroenterol, 2015, 21( 22): 6769- 6784. DOI: 10.3748/wjg.v21.i22.6769. [9] MARUYAMA H, OKUGAWA H, TAKAHASHI M, et al. De novo portal vein thrombosis in virus-related cirrhosis: Predictive factors and long-term outcomes[J]. Am J Gastroenterol, 2013, 108( 4): 568- 574. DOI: 10.1038/ajg.2012.452. [10] LYU SC, HE Q. Research progress in perioperative management of portal vein thrombosis in liver transplantation[J]. Organ Transplantation, 2024, 15( 1): 26- 32. DOI: 10.3969/j.issn.1674-7445.2023185.吕少诚, 贺强. 肝移植围手术期门静脉血栓管理的研究进展[J]. 器官移植, 2024, 15( 1): 26- 32. DOI: 10.3969/j.issn.1674-7445.2023185. [11] ZHOU N, FENG WJ, WANG YL, et al. Predictive factors and prognosis analysis of liver cirrhosis complicated with portal vein thrombosis[C/OL]// The 12th National Conference on Difficult and Severe Liver Diseases. Dalian, 2023: 123- 124. DOI: 10.26914/c.cnkihy.2023.022609.周宁, 冯文娟, 汪雨露, 等. 肝硬化并发门静脉血栓的预测因素及预后分析[C/OL]// 第12届全国疑难及重症肝病大会. 大连, 2023: 123- 124. DOI: 10.26914/c.cnkihy.2023.022609. [12] RUAN FM, LI BM. Risk factors for the formation of portal vein thrombosis in patients with liver cirrhosis[J]. J Clin Hepatol, 2020, 36( 1): 182- 185. DOI: 10.3969/j.issn.1001-5256.2020.01.043.阮芳鸣, 李弼民. 肝硬化门静脉血栓形成的危险因素[J]. 临床肝胆病杂志, 2020, 36( 1): 182- 185. DOI: 10.3969/j.issn.1001-5256.2020.01.043. [13] CHEN S, YANG CQ. Pathogenesis and risk factors of portal vein thrombosis[J]. J Pract Hepatol, 2019, 22( 6): 761- 764. DOI: 10.3969/j.issn.1672-5069.2019.06.001.陈帅, 杨长青. 门静脉血栓病因及危险因素研究进展[J]. 实用肝脏病杂志, 2019, 22( 6): 761- 764. DOI: 10.3969/j.issn.1672-5069.2019.06.001. [14] NERY F, CARNEIRO P, CORREIA S, et al. Systemic inflammation as a risk factor for portal vein thrombosis in cirrhosis: A prospective longitudinal study[J]. Eur J Gastroenterol Hepatol, 2021, 33( 1 S Suppl 1): e108- e113. DOI: 10.1097/MEG.0000000000001982. [15] STINE JG, PRAKASH S, NORTHUP PG. Portal vein thrombosis after hepatitis C eradication with direct acting antiviral therapy[J]. Liver Int, 2018, 38( 1): 185- 186. DOI: 10.1111/liv.13537. [16] BASILI S, CARNEVALE R, NOCELLA C, et al. Serum albumin is inversely associated with portal vein thrombosis in cirrhosis[J]. Hepatol Commun, 2019, 3( 4): 504- 512. DOI: 10.1002/hep4.1317. [17] WANG LH, WANG RX, ZHAO ZY, et al. Clinical significance of portal vein thrombosis in patients with HBV related decompensated liver cirrhosis[J]. Chin Hepatol, 2022, 27( 8): 874- 876. DOI: 10.14000/j.cnki.issn.1008-1704.2022.08.007.王利慧, 王荣希, 赵泽源, 等. 失代偿期乙型肝炎肝硬化患者门静脉血栓形成的临床意义[J]. 肝脏, 2022, 27( 8): 874- 876. DOI: 10.14000/j.cnki.issn.1008-1704.2022.08.007. [18] FAN J, GAO XF, WANG ZL, et al. Risk factors and awareness of deep vein thrombosis among outpatients in Shanghai community hospitals:a multi—center study[J]. Chin J Geriatr Hear Brain Vessel Dis, 2023, 25( 12): 1289- 1292. DOI: 10.3969/j.issn.1009-0126.2023.12.014.樊剑, 高雪峰, 王振雷, 等. 上海市多中心社区医院门诊患者深静脉血栓风险因素分析及认知水平评估[J]. 中华老年心脑血管病杂志, 2023, 25( 12): 1289- 1292. DOI: 10.3969/j.issn.1009-0126.2023.12.014. [19] LIU SS, YU XH, QIN JW. Changing trend of the disease burden of liver cirrhosis in China from 1990 to 2019[J]. J Clin Hepatol, 2024, 40( 4): 726- 733. DOI: 10.12449/JCH240414.刘珊山, 于晓辉, 秦建伟. 1990—2019年中国肝硬化疾病负担变化趋势分析[J]. 临床肝胆病杂志, 2024, 40( 4): 726- 733. DOI: 10.12449/JCH240414. [20] YOU H, WANG F, LI T, et al. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. J Clin Transl Hepatol, 2023, 11( 6): 1425- 1442. DOI: 10.14218/jcth.2023.00320. [21] WENG FW, GUO LY, LI QW, et al. Change in the expression of peripheral blood lymphocyte subsets during disease progression in patients with chronic hepatitis B virus infection[J]. J Clin Hepatol, 2020, 36( 1): 65- 69. DOI: 10.3969/j.issn.1001-5256.2020.01.015.翁奉武, 郭丽颖, 李秋伟, 等. 外周血淋巴细胞亚群在慢性HBV感染过程中表达的变化分析[J]. 临床肝胆病杂志, 2020, 36( 1): 65- 69. DOI: 10.3969/j.issn.1001-5256.2020.01.015. [22] HAN JB, SHU QH, ZHANG YF, et al. Predictive value of inflammation biomarkers in patients with portal vein thrombosis[J]. J Clin Transl Hepatol, 2021, 9( 3): 384- 391. DOI: 10.14218/JCTH.2020.00159. [23] HUANG XQ, FAN XW, ZHANG R, et al. Systemic inflammation and portal vein thrombosis in cirrhotic patients with gastroesophageal varices[J]. Eur J Gastroenterol Hepatol, 2020, 32( 3): 401- 405. DOI: 10.1097/MEG.0000000000001526. [24] PIANTANIDA E, IPPOLITO S, GALLO D, et al. The interplay between thyroid and liver: Implications for clinical practice[J]. J Endocrinol Invest, 2020, 43( 7): 885- 899. DOI: 10.1007/s40618-020-01208-6. [25] ZHANG JY, TANG YM. Novel advances in the relationship between chronic liver disease and thyroid disorder[J]. Chin Gen Pract, 2021, 24( 33): 4281- 4286..张静怡, 唐映梅. 慢性肝病与甲状腺功能障碍关系研究进展[J]. 中国全科医学, 2021, 24( 33): 4281- 4286. [26] HARTL L, SIMBRUNNER B, JACHS M, et al. Lower free triiodothyronine(fT3) levels in cirrhosis are linked to systemic inflammation, higher risk of acute-on-chronic liver failure, and mortality[J]. JHEP Rep, 2023, 6( 1): 100954. DOI: 10.1016/j.jhepr.2023.100954. [27] SINGH B, GUPTA, HOODA N, et al. Chronic liver disease secondary to chronic budd chiari causing osteomalacia leading to pathological fractures[J]. J Assoc Physicians India, 2023, 71( 1): 1. [28] SALLOUM-ASFAR S, BOELEN A, REITSMA PH, et al. The immediate and late effects of thyroid hormone(triiodothyronine) on murine coagulation gene transcription[J]. PLoS One, 2015, 10( 5): e0127469. DOI: 10.1371/journal.pone.0127469. [29] VISWANATHAN G, BALASUBRAMANIAM K, HARDY R, et al. Blood thrombogenicity is independently associated with serum TSH levels in post-non-ST elevation acute coronary syndrome[J]. J Clin Endocrinol Metab, 2014, 99( 6): E1050- E1054. DOI: 10.1210/jc.2013-3062. [30] KOVÁŘOVÁ M, KOLLER T, ŠTVRTINOVÁ V, et al. Thyroid-stimulating hormone concentration as an independent risk factor of venous thromboembolism regardless of thyroid function[J]. Endokrynol Pol, 2015, 66( 6): 474- 479. DOI: 10.5603/EP.2015.0058. [31] SARAIVA M, O’GARRA A. The regulation of IL-10 production by immune cells[J]. Nat Rev Immunol, 2010, 10( 3): 170- 181. DOI: 10.1038/nri2711. [32] RUTZ S, OUYANG WJ. Regulation of interleukin-10 and interleukin-22 expression in T helper cells[J]. Curr Opin Immunol, 2011, 23( 5): 605- 612. DOI: 10.1016/j.coi.2011.07.018. [33] SARAIVA M, VIEIRA P, O’GARRA A. Biology and therapeutic potential of interleukin-10[J]. J Exp Med, 2020, 217( 1): e20190418. DOI: 10.1084/jem.20190418. [34] HUANG L, YU QS, WANG JJ. Association between changes in splanchnic hemodynamics and risk factors of portal venous system thrombosis after splenectomy with periesophagogastric devascularization[J]. Med Sci Monit, 2018, 24: 4355- 4362. DOI: 10.12659/msm.909403. [35] CHEN YJ, WAN XY, LI Y, et al. Risk factors for portal vein thrombosis in cirrhotic patients and the influences of anticoagulation on esophagogastric variceal bleeding[J]. J Chin Physician, 2019, 21( 12): 1808- 1812, 1816. DOI: 10.3760/cma.j.issn.1008-1372.2019.12.013.陈艳洁, 万欣宇, 李媛, 等. 肝硬化者并门静脉血栓形成的危险因素及抗凝治疗对其上消化道出血的影响[J]. 中国医师杂志, 2019, 21( 12): 1808- 1812, 1816. DOI: 10.3760/cma.j.issn.1008-1372.2019.12.013. [36] XU DQ, YANG JH. Correlational study on portal vein thrombosis of liver cirrhosis[J]. Chin J Hepatol, 2020, 28( 7): 573- 579. DOI: 10.3760/cma.j.cn501113-20190404-00107.许丹青, 杨晋辉. 肝硬化门静脉血栓形成的相关研究[J]. 中华肝脏病杂志, 2020, 28( 7): 573- 579. DOI: 10.3760/cma.j.cn501113-20190404-00107. [37] ZHANG M. Portal vein thrombosis in cirrhotic patients: The states of the art[J]. J Pract Hepatol, 2024, 27( 1): 11- 15. DOI: 10.3969/j.issn.1672-5069.2024.01.004.张明. 肝硬化门静脉血栓形成诊治新进展[J]. 实用肝脏病杂志, 2024, 27( 1): 11- 15. DOI: 10.3969/j.issn.1672-5069.2024.01.004. [38] LIU F, ZHONG H, WEI W, et al. Analysis on clinical features and risk factors of liver cirrhosis portal vein thrombosis associated with metabolism-associated fatty liver disease[J]. Chongqing Med J, 2024, 53( 7): 1045- 1049. DOI: 10.3969/j.issn.1671-8348.2024.07.016.刘菲, 钟黄, 魏尉, 等. MAFLD相关肝硬化门静脉血栓形成的临床特征及危险因素分析[J]. 重庆医学, 2024, 53( 7): 1045- 1049. DOI: 10.3969/j.issn.1671-8348.2024.07.016. [39] ZHU HY. Nomogram predictive model analysis and validation of hepatic cirrhosis complicated with portal venous thrombosis[D]. Wulumuqi: Xinjiang Medical University, 2023.朱海艳. 肝硬化门静脉血栓形成的列线图预测模型分析及验证[D]. 乌鲁木齐: 新疆医科大学, 2023. [40] XING YY, TIAN ZB, JIANG YP, et al. A practical nomogram based on systemic inflammatory markers for predicting portal vein thrombosis in patients with liver cirrhosis[J]. Ann Med, 2022, 54( 1): 302- 309. DOI: 10.1080/07853890.2022.2028893. -



 PDF下载 ( 2198 KB)
PDF下载 ( 2198 KB)

 下载:
下载: