乙型肝炎核心抗体定量对国际标准化比值正常的乙型肝炎肝硬化失代偿期患者预后的预测价值
DOI: 10.12449/JCH250814
Value of quantitative hepatitis B core antibody in predicting the prognosis of decompensated hepatitis B cirrhosis patients with normal international normalized ratio
-
摘要:
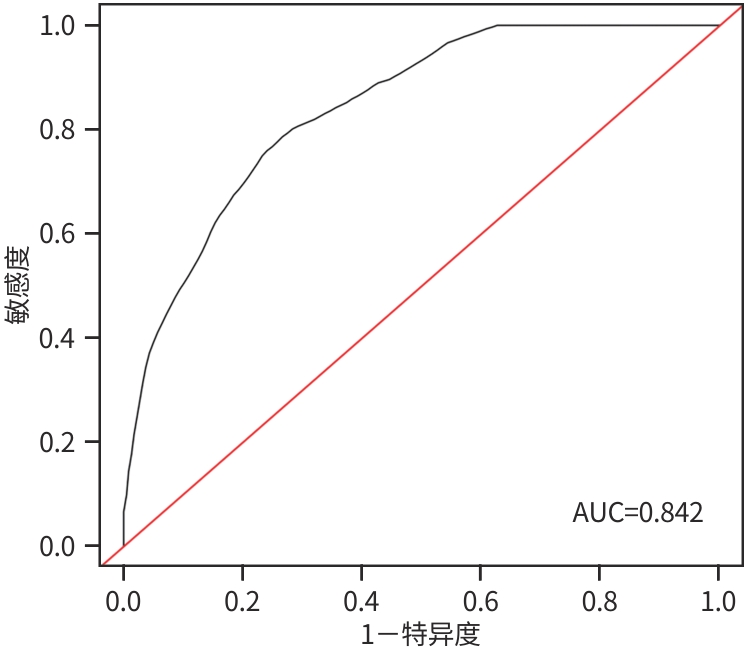
目的 测定血清乙型肝炎核心抗体定量(qAnti-HBc)在国际标准化比值(INR)正常的乙型肝炎肝硬化失代偿期患者中的水平,探讨其在该目标人群中预后的价值。 方法 纳入2018年10月1日—2021年4月1日在天津市第三中心医院诊治的INR正常的乙型肝炎肝硬化失代偿期患者120例,收集基线指标,测定血清qAnti-HBc水平,随访预后情况。根据预后,分为生存组和死亡组。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用单因素和多因素Cox回归分析筛选影响预后的候选变量,构建该人群预后模型,绘制受试者操作特征曲线(ROC曲线)。 结果 入组患者的平均随访时间为(32.17±13.09)个月,生存组99例(82.5%),死亡组21例(17.5%),生存组与死亡组比较,性别(χ2=2.151,P=0.014)、年龄(t=-3.218,P=0.003)、TBil(Z=-0.901,P=0.027)、Alb(t=3.353,P=0.001)、Child-Pugh分级(χ2=1.144,P=0.010)差异均有统计学意义。单因素及多因素Cox分析提示,血清qAnti-HBc(HR=0.57,95%CI:0.32~1.00,P=0.043)、年龄(HR=1.06,95%CI:1.00~1.12,P=0.044)、性别(HR=3.82,95%CI:1.46~10.00,P=0.006)、血小板(HR=0.98,95%CI:0.97~1.00,P=0.037)、Alb(HR=0.87,95%CI:0.79~0.95,P=0.002)为影响INR正常的乙型肝炎肝硬化失代偿期患者预后的独立风险预测因素,据此建立预测模型为:h(t,x)/h0(t)=exp(1.34X1+0.06X2-0.14X3-0.02X4-0.57X5),其中X1为性别,X2为年龄,X3为Alb,X4为PLT,X5为qAnti-HBc。该预测模型ROC曲线下面积为0.842,其敏感度为0.79,特异度为0.73,预测模型的C指数为0.85。 结论 在INR正常(0.8~1.2)的乙型肝炎肝硬化失代偿期患者中,血清qAnti-HBc为患者死亡的独立风险预测因素之一,其联合其他指标建立的预测模型可有效预测预后。 Abstract:Objective To determine the serum level of quantitative hepatitis B core antibody (qAnti-HBc) in decompensated hepatitis B cirrhosis patients with normal international normalized ratio (INR), and to investigate its prognostic value in this target population. Methods A total of 120 decompensated hepatitis B cirrhosis patients with normal INR who were diagnosed and treated in Tianjin Third Central Hospital from October 1, 2018 to April 1, 2021 were enrolled. Baseline indicators were collected, the serum level of qAnti-HBc was measured, and the prognosis was followed up. According to the prognosis, the patients were divided into survival group and death group. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between groups. Univariate and multivariate Cox regression analyses were used to investigate the candidate variables affecting the prognosis and establish a prognostic model for this population, and the receiver operating characteristic (ROC) curve was plotted. Results The patients enrolled were followed up for 32.17±13.09 months, and there were 99 patients (82.5%) in the survival group and 21 patients (17.5%) in the death group. There were significant differences between the survival group and the death group in sex (χ2=2.151, P=0.014), age (t=-3.218, P=0.003), total bilirubin (Z=-0.901, P=0.027), albumin (t=3.353, P=0.001), and Child-Pugh class (χ2=1.144, P=0.010). The univariate and multivariate Cox regression analyses showed that serum qAnti-HBc (hazard ratio [HR]=0.57, 95% confidence interval [CI]: 0.32 — 1.00, P=0.043), age (HR=1.06, 95%CI:1.00 — 1.12, P=0.044), sex (HR=3.82, 95%CI: 1.46 — 10.00, P=0.006), platelet count (HR=0.98, 95%CI: 0.97 — 1.00, P=0.037), and albumin (HR=0.86, 95%CI: 0.79 — 0.95, P=0.002) were independent risk predictive factors for the prognosis of decompensated hepatitis B cirrhosis patients with normal INR. Based on these factors, a predictive model was established as h(t, x)/h0(t)=exp(1.34X1+0.06X2-0.14X3-0.02X4-0.57X5), where X1=sex, X2=age, X3=albumin, X4=platelet count, X5=qAnti-HBc. This predictive model had an area under the ROC curve of 0.842, with a sensitivity of 0.79, a specificity of 0.73, and a C-index of 0.85. Conclusion In decompensated hepatitis B cirrhosis patients with normal INR (0.8 — 1.2), serum qAnti-HBc is one of the independent risk predictive factors for death, and the predictive model established based on the combination of serum qAnti-HBc and other indicators can effectively predict the prognosis of patients. -
Key words:
- Hepatitis B Core Antibody /
- Liver Cirrhosis /
- Prognosis /
- Risk Factors
-
表 1 INR正常的乙型肝炎肝硬化失代偿期患者生存组与死亡组的基线特征
Table 1. Baseline characteristics of death versus survival group in decompensated hepatitis B cirrhosis with normal INR patients
指标 生存组(n=99) 死亡组(n=21) 统计值 P值 性别[例(%)] χ2=2.151 0.014 男 81(81.82) 12(57.14) 女 18(18.18) 9(42.86) 年龄(岁) 54.37±10.51 62.29±8.79 t=-3.218 0.003 乙型肝炎家族史[例(%)] χ2=0.092 0.999 有 29(29.29) 6(28.57) 无 70(70.71) 15(71.43) 乙型肝炎病史(年) 6.00(0.83~14.50) 7.00(3.00~12.00) Z=-0.623 0.416 肝癌家族史[例(%)] χ2=0.251 0.956 有 5(5.05) 1(4.76) 无 94(94.95) 20(95.24) 2型糖尿病[例(%)] χ2=0.572 0.670 有 18(18.18) 3(14.29) 无 81(81.82) 18(85.71) 入组前抗病毒治疗[例(%)] χ2=0.887 0.342 有 87(87.88) 16(76.19) 无 12(12.12) 5(23.81) 入组前抗病毒时间[例(%)] χ2=0.358 0.734 ≥1年 81(81.82) 14(66.67) <1年 18(18.18) 7(33.33) 抗病毒治疗药物[例(%)] χ2=2.950 0.451 未抗病毒 16(16.16) 2(9.52) 核苷类药物 64(64.65) 16(76.19) 核苷酸类药物 11(11.11) 2(9.52) 核苷(酸)类联合 8(8.08) 1(4.76) INR 1.19±0.08 1.21±0.05 t=-2.551 0.349 ALT(U/L) 25.00(17.00~37.00) 19.00(13.00~27.00) Z=1.346 0.537 AST(U/L) 32.00(22.00~48.00) 28.00(21.00~44.00) Z=1.247 0.559 TBil(μmol/L) 20.10(14.55~28.95) 24.30(21.10~43.10) Z=-0.901 0.027 Na(mmol/L) 139.44±2.85 139.62±3.30 t=-0.244 0.808 Cr(μmol/L) 67.00(61.00~75.00) 60.00(49.50~79.00) Z=-0.638 0.201 PLT(×109/L) 62.00(43.00~96.00) 60.00(43.00~76.00) Z=2.277 0.134 Alb(g/L) 36.92±5.65 32.40±5.40 t=3.353 0.001 HBsAg(log10 IU/mL) 2.76(1.39~3.30) 2.21(1.30~3.14) Z=0.700 0.511 HBeAg[例(%)] χ2=0.264 0.797 阳性 26(26.26) 5(23.81) 阴性 73(73.74) 16(76.19) HBV DNA(log10 IU/mL) 2.00(1.70~2.78) 2.00(1.70~3.77) Z=-0.363 0.772 qAnti-HBc(log10 IU/mL) 3.34±0.93 2.90±0.84 t=1.926 0.057 Child-Pugh分级[例(%)] χ2=1.144 0.010 A级 70(70.71) 8(38.10) B级 27(27.27) 11(52.38) C级 2(2.02) 2(9.52) 表 2 INR正常的乙型肝炎肝硬化失代偿期患者发生死亡风险的Cox回归分析
Table 2. Cox regression of death events in decompensated hepatitis B cirrhosis with normal INR patients
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 乙型肝炎家族史(是=1,否=0) 0.93 0.36~2.40 0.875 乙型肝炎病史(年) 1.02 0.98~1.06 0.385 肝癌家族史(是=1,否=0) 0.98 0.13~7.33 0.985 2型糖尿病(是=1,否=0) 0.82 0.24~2.28 0.743 入组前抗病毒治疗(是=1,否=0) 1.37 0.56~3.35 0.496 抗病毒治疗方案 未抗病毒 1.00 核苷类药物 1.63 0.37~7.12 0.518 核苷酸类药物 1.20 0.17~8.56 0.853 核苷(酸)类联合 0.01 0.00~Inf 0.998 入组前抗病毒时间(>1年=1,≤1年=0) 1.08 0.32~3.71 0.898 ALT(U/L) 0.98 0.95~1.01 0.218 AST(U/L) 1.00 0.98~1.01 0.477 TBil(μmol/L) 1.00 0.99~1.01 0.544 Na(mmol/L) 1.02 0.87~1.19 0.814 HBsAg(log10 IU/mL) 0.67 0.20~2.28 0.519 HBeAg(阳性=1,阴性=0) 0.85 0.31~2.35 0.759 HBV DNA(log10 IU/mL) 1.40 0.56~3.49 0.466 Cr(μmol/L) 1.01 1.00~ 1.01 0.090 1.00 1.00~1.01 0.056 Child-Pugh分级 A级 1.00 B级 3.32 1.32~8.33 0.001 2.50 0.83~7.48 0.102 C级 9.08 1.87~44.05 0.006 1.54 0.13~18.66 0.736 性别(男性=1,女性=2) 2.84 1.19~6.77 0.019 3.82 1.46~10.00 0.006 年龄(岁) 1.09 1.03~1.14 <0.001 1.06 1.00~1.12 0.044 PLT(×109/L) 0.99 0.98~1.00 0.099 0.98 0.97~1.00 0.037 Alb(g/L) 0.88 0.81~0.95 <0.001 0.87 0.79~0.95 0.002 qAnti-HBc(log10 IU/mL) 0.57 0.33~1.00 0.049 0.57 0.32~1.00 0.043 -
[1] GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 796- 829. DOI: 10.1016/S2468-1253(22)00124-8. [2] European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69( 2): 406- 460. DOI: 10.1016/j.jhep.2018.03.024. [3] GINÈS P, KRAG A, ABRALDES JG, et al. Liver cirrhosis[J]. Lancet, 2021, 398( 10308): 1359- 1376. DOI: 10.1016/s0140-6736(21)01374-x. [4] D’AMICO G, GARCIA-TSAO G, PAGLIARO L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies[J]. J Hepatol, 2006, 44( 1): 217- 231. DOI: 10.1016/j.jhep.2005.10.013. [5] JALAN R, D’AMICO G, TREBICKA J, et al. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis[J]. J Hepatol, 2021, 75( Suppl 1): S14- S26. DOI: 10.1016/j.jhep.2021.01.018. [6] JIA W, SONG LW, FANG YQ, et al. Antibody to hepatitis B core antigen levels in the natural history of chronic hepatitis B: A prospective observational study[J]. Medicine(Baltimore), 2014, 93( 29): e322. DOI: 10.1097/MD.0000000000000322. [7] HOU FQ, SONG LW, YUAN Q, et al. Quantitative hepatitis B core antibody level is a new predictor for treatment response in HBeAg-positive chronic hepatitis B patients receiving peginterferon[J]. Theranostics, 2015, 5( 3): 218- 226. DOI: 10.7150/thno.10636. [8] CHI H, LI ZD, HANSEN BE, et al. Serum level of antibodies against hepatitis B core protein is associated with clinical relapse after discontinuation of nucleos(t)ide analogue therapy[J]. Clin Gastroenterol Hepatol, 2019, 17( 1): 182- 191. e 1. DOI: 10.1016/j.cgh.2018.05.047. [9] ZHANG ZQ, SHI BS, LU W, et al. Quantitative anti-HBc in liver pathological states in patients with chronic hepatitis B virus infection[J]. Can J Infect Dis Med Microbiol, 2019, 2019: 6545642. DOI: 10.1155/2019/6545642. [10] LI MR, ZHENG HW, MA SM, et al. Correlations between serum hepatitis B surface antigen and hepatitis B core antibody titers and liver fibrosis in treatment-naïve CHB patients[J]. J Chin Med Assoc, 2018, 81( 12): 1052- 1059. DOI: 10.1016/j.jcma.2018.05.007. [11] WU Z, DONG XQ, WANG GQ, et al. Clinical noninvasive markers for antiviral therapy decision in chronic hepatitis B with alanine aminotransferase less than two times upper limit of normal[J]. J Viral Hepat, 2019, 26( 2): 287- 296. DOI: 10.1111/jvh.13030. [12] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update[J]. J Clin Hepatol, 2015, 31( 12): 1941- 1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31( 12): 1941- 1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [13] BEDOSSA P, POYNARD T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group[J]. Hepatology, 1996, 24( 2): 289- 293. DOI: 10.1002/hep.510240201. [14] Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China(2017 Edition)[J]. Chin J Diabetes, 2018, 10( 1): 4- 67. DOI: 10.3760/cma.j.issn.1674-5809.2018.01.003.中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2017年版)[J]. 中华糖尿病杂志, 2018, 10( 1): 4- 67. DOI: 10.3760/cma.j.issn.1674-5809.2018.01.003. [15] LI A, YUAN Q, HUANG ZY, et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody[J]. Clin Vaccine Immunol, 2010, 17( 3): 464- 469. DOI: 10.1128/CVI.00457-09. [16] LIAW YF, CHU CM. Hepatitis B virus infection[J]. Lancet, 2009, 373( 9663): 582- 592. DOI: 10.1016/S0140-6736(09)60207-5. [17] LI J, GONG QM, XIE PL, et al. Prognostic value of anti-HBc quantification in hepatitis B virus related acute-on-chronic liver failure[J]. J Gastroenterol Hepatol, 2021, 36( 5): 1291- 1299. DOI: 10.1111/jgh.15310. [18] YUAN Q, SONG LW, LIU CJ, et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients[J]. Gut, 2013, 62( 1): 182- 184. DOI: 10.1136/gutjnl-2012-302656. [19] FAN R, SUN J, YUAN Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues[J]. Gut, 2016, 65( 2): 313- 320. DOI: 10.1136/gutjnl-2014-308546. [20] XU JH, SONG LW, LI N, et al. Baseline hepatitis B core antibody predicts treatment response in chronic hepatitis B patients receiving long-term entecavir[J]. J Viral Hepat, 2017, 24( 2): 148- 154. DOI: 10.1111/jvh.12626. [21] DEZANET LNC, MAYLIN S, GABASSI A, et al. Kinetics of hepatitis B core-related antigen and anti-hepatitis B core antibody and their association with serological response in human immunodeficiency virus-hepatitis B coinfection[J]. J Infect Dis, 2020, 221( 11): 1826- 1837. DOI: 10.1093/infdis/jiaa013. [22] LI J, MAO RC, LI XL, et al. A novel noninvasive index for the prediction of moderate to severe fibrosis in chronic hepatitis B patients[J]. Dig Liver Dis, 2018, 50( 5): 482- 489. DOI: 10.1016/j.dld.2017.12.028. [23] FAN R, PAPATHEODORIDIS G, SUN J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis[J]. J Hepatol, 2020, 73( 6): 1368- 1378. DOI: 10.1016/j.jhep.2020.07.025. [24] FENG XS, WANG G, LI N, et al. The association between fasting blood glucose and the risk of primary liver cancer in Chinese males: A population-based prospective study[J]. Br J Cancer, 2017, 117( 9): 1405- 1411. DOI: 10.1038/bjc.2017.296. [25] CHEN RC, CAI YJ, WU JM, et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis[J]. J Viral Hepat, 2017, 24( 3): 238- 245. DOI: 10.1111/jvh.12638. [26] LI SM, LIU B, PENG SL, et al. Correlation between HBV DNA load, serum IL-2R, GP73, miR-21 and HBVM expression pattern, liver histopathological changes in patients with chronic hepatitis B[J]. J Clin Exp Med, 2024, 23( 10): 1017- 1021. DOI: 10.3969/j.issn.1671-4695.2024.10.003.李述美, 刘冰, 彭思璐, 等. 慢性乙型肝炎患者HBV DNA载量及血清IL-2R、GP73、miR-21与HBVM表达模式、肝组织病理学改变的相关性[J]. 临床和实验医学杂志, 2024, 23( 10): 1017- 1021. DOI: 10.3969/j.issn.1671-4695.2024.10.003. [27] SONG LW, LIU PG, LIU CJ, et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection[J]. Clin Microbiol Infect, 2015, 21( 2): 197- 203. DOI: 10.1016/j.cmi.2014.10.002. [28] BERNSMEIER C, van der MERWE S, PÉRIANIN A. Innate immune cells in cirrhosis[J]. J Hepatol, 2020, 73( 1): 186- 201. DOI: 10.1016/j.jhep.2020.03.027. [29] YE GF, CHEN CC, ZHOU YJ, et al. Anti-HBc mirrors the activation of HBV-specific CD8+ T cell immune response and exhibits a direct effect on HBV control[J]. Antiviral Res, 2024, 230: 105975. DOI: 10.1016/j.antiviral.2024.105975. [30] TANG LB, CHEN CC, GAO XP, et al. Interleukin 21 reinvigorates the antiviral activity of hepatitis B virus(HBV)-specific CD8+ T cells in chronic HBV infection[J]. J Infect Dis, 2019, 219( 5): 750- 759. DOI: 10.1093/infdis/jiy576. -



 PDF下载 ( 916 KB)
PDF下载 ( 916 KB)

 下载:
下载:


