基于CT三维重建技术探讨脾脏体积与非酒精性脂肪性肝病的关系
DOI: 10.12449/JCH250813
Relationship between spleen volume and non-alcoholic fatty liver disease by three-dimensional computed tomography reconstruction
-
摘要:
目的 探讨脾脏体积与非酒精性脂肪性肝病(NAFLD)发病风险的相关性,并进一步探究脾脏体积与NAFLD的因果关系。 方法 选取2022年11月—2023年11月在西安交通大学第二附属医院行腹部增强CT检查的个体,包括90例NAFLD患者和47例健康对照者。采用两阶段由粗到细的图像分割方法,通过构建深度学习网络模型,对脾脏进行三维重建。计量资料两组间比较采用成组t检验或Mann-Whitney U检验;计数资料两组间比较采用χ2检验。采用Pearson相关或Spearman秩相关分析脾脏体积与肝功能指标的相关性。采用多因素Logistic回归分析NAFLD发生的影响因素。此外,进一步采用双样本孟德尔随机化(MR)探讨脾脏体积与NAFLD之间是否存在因果关联,逆方差加权法(IVW)为MR主要研究方法。 结果 NAFLD患者脾脏体积显著大于健康对照者[(272.93±104.16) cm3 vs (204.37±81.20) cm3,P<0.001]。Spearman秩相关分析显示,NAFLD患者脾脏体积与肝脂肪变性指数(HIS)(rs =0.422,P<0.001)和GGT(rs =0.211,P=0.047)呈正相关。多因素Logistic回归模型结果显示,脾脏体积是NAFLD发生的独立危险因素(OR=1.01,95% CI:1.00~1.02,P=0.049);IVW结果显示,脾脏体积与NAFLD存在因果关系(OR=1.16,95%CI:1.05~1.28,P=0.005)。 结论 脾脏体积增大可能是NAFLD发生、发展的一个危险因素,具体机制仍需进一步深入研究。 Abstract:Objective To investigate the association of spleen volume with the risk of non-alcoholic fatty liver disease (NAFLD) as well as their causal relationship. Methods We included 90 NAFLD cases and 47 healthy controls who had received contrast-enhanced computed tomography (CT) scan of the abdomen at the Second Affiliated Hospital of Xi’an Jiaotong University from November 2022 to November 2023. We conducted three-dimensional reconstruction of the spleen through a deep learning network model using a two-stage coarse-to-fine segmentation approach. We compared the two groups using the two-sample t test or Mann-Whitney U test for continuous data and using the chi-square test for categorical data; evaluated the correlation between spleen volume and liver function indicators through Pearson correlation or Spearman rank correlation analyses; determined the factors influencing the development of NAFLD through multivariable Logistic regression analysis; and further assessed the casual relationship between spleen volume and NAFLD using the inverse variance-weighted two-sample Mendelian randomization (IVW-MR) method. Results Spleen volume was significantly larger in NAFLD cases than in controls (272.93±104.16 vs 204.37±81.20 cm3, P<0.001). The Spearman rank correlation analysis showed that spleen volume was positively correlated with the hepatic steatosis index (rs =0.422, P<0.001) and gamma-glutamyl transferase levels (rs =0.211, P=0.047) in patients with NAFLD. The multivariable Logistic regression analysis indicated that spleen volume was an independent risk factor for the development of NAFLD (odds ratio [OR]=1.01, 95% confidence interval [CI]: 1.00 — 1.02, P=0.049). The IVW-MR analysis detected a causal relationship between spleen volume and NAFLD (OR=1.16, 95%CI: 1.05 — 1.28, P=0.005). Conclusion Increased spleen volume may be a risk factor for the development and progression of NAFLD. Further studies are still needed to investigate the specific mechanism. -
表 1 NAFLD组和健康对照组基线及临床特征比较
Table 1. Baseline demographic and clinical characteristics between NAFLD patients and controls
指标 NAFLD组(n=90) 健康对照组(n=47) 统计值 P值 脾脏体积(cm3) 272.93±104.16 204.37±81.20 t=-3.929 <0.001 年龄(岁) 47.34±12.42 55.51±14.21 t=3.475 0.001 男[例(%)] 59(65.56) 26(55.32) χ2=1.374 0.241 BMI(kg/m2) 26.71±3.44 23.59±2.69 t=-4.208 0.005 HSI 37.29(34.50~41.29) 30.88(28.92~33.19) Z=-5.265 <0.001 eGFR(mL·min-1·1.73m-²) 108.09(95.31~114.71) 107.73(98.30~115.69) Z=-0.467 0.641 血脂异常[例(%)] 67(74.44) 18(38.30) χ2=17.131 <0.001 ALT(U/L) 34.00(22.50~45.00) 16.00(12.00~22.40) Z=-6.264 <0.001 AST(U/L) 27.00(21.50~36.50) 20.00(16.00~26.00) Z=-3.950 <0.001 GGT(U/L) 39.00(27.50~74.50) 18.00(14.00~25.00) Z=-6.300 <0.001 ALP(U/L) 81.00(67.50~93.00) 70.00(60.00~96.00) Z=-1.321 0.187 TP(g/L) 70.20±5.82 68.08±6.82 t=-1.908 0.059 Alb(g/L) 44.32±4.21 41.79±4.58 t=-3.238 0.002 Glb(g/L) 25.88±3.87 26.29±4.09 t=0.575 0.566 A/G 1.75±0.29 1.62±0.27 t=-2.487 0.014 表 2 脾脏体积与肝功能指标的相关性分析
Table 2. Correlations of spleen volume with indicators of liver function
指标 总人群(n=137) NALFD组(n=90) 健康对照组(n=47) 相关系数 P值 相关系数 P值 相关系数 P值 HSI 0.338 0.001 0.422 <0.001 -0.138 0.501 ALT(U/L) 0.206 0.016 0.198 0.063 -0.087 0.560 AST(U/L) 0.066 0.445 0.058 0.592 -0.284 0.053 GGT(U/L) 0.226 0.008 0.211 0.047 -0.135 0.364 ALP(U/L) 0.139 0.107 0.104 0.334 0.070 0.638 TP(g/L) 0.103 0.231 0.027 0.801 0.115 0.441 Alb(g/L) 0.118 0.172 0.022 0.835 0.061 0.686 Glb(g/L) 0.029 0.734 0.016 0.880 0.124 0.407 A/G 0.064 0.462 0.034 0.755 -0.105 0.483 表 3 Logistic回归分析NAFLD的影响因素
Table 3. Logistic regression analysis of influencing factors for NAFLD
变量 单因素Logistic回归分析 多因素Logistic回归分析 OR(95%CI) P值 OR(95%CI) P值 脾脏体积(cm3) 1.01(1.00~1.01) <0.001 1.01(1.00~1.02) 0.049 年龄(岁) 0.95(0.93~0.98) 0.001 0.99(0.94~1.04) 0.693 性别(0=男;1=女) 1.54(0.75~3.16) 0.242 BMI(kg/m2) 1.37(1.16~1.62) <0.001 0.69(0.43~1.09) 0.112 HSI 1.34(1.17~1.53) <0.001 1.59(1.05~2.40) 0.028 血脂异常(0=否;1=是) 8.80(3.66~21.13) <0.001 3.98(1.34~11.86) 0.013 eGFR(mL·min-1·1.73 m-²) 0.99(0.97~1.01) 0.479 ALT(U/L) 1.10(1.05~1.14) <0.001 0.99(0.89~1.10) 0.826 AST(U/L) 1.08(1.04~1.14) 0.001 1.09(0.97~1.23) 0.133 GGT(U/L) 1.01(1.00~1.02) 0.088 ALP(U/L) 1.00(0.99~1.01) 0.619 TP(g/L) 1.06(0.98~1.12) 0.061 Alb(g/L) 1.14(1.05~1.25) 0.003 1.03(0.90~1.17) 0.668 Glb(g/L) 0.97(0.89~1.07) 0.564 A/G 5.13(1.35~19.47) 0.016 7.40(0.75~72.63) 0.086 表 4 脾脏体积与NAFLD的MR结果
Table 4. The association between spleen volume and NAFLD by MR
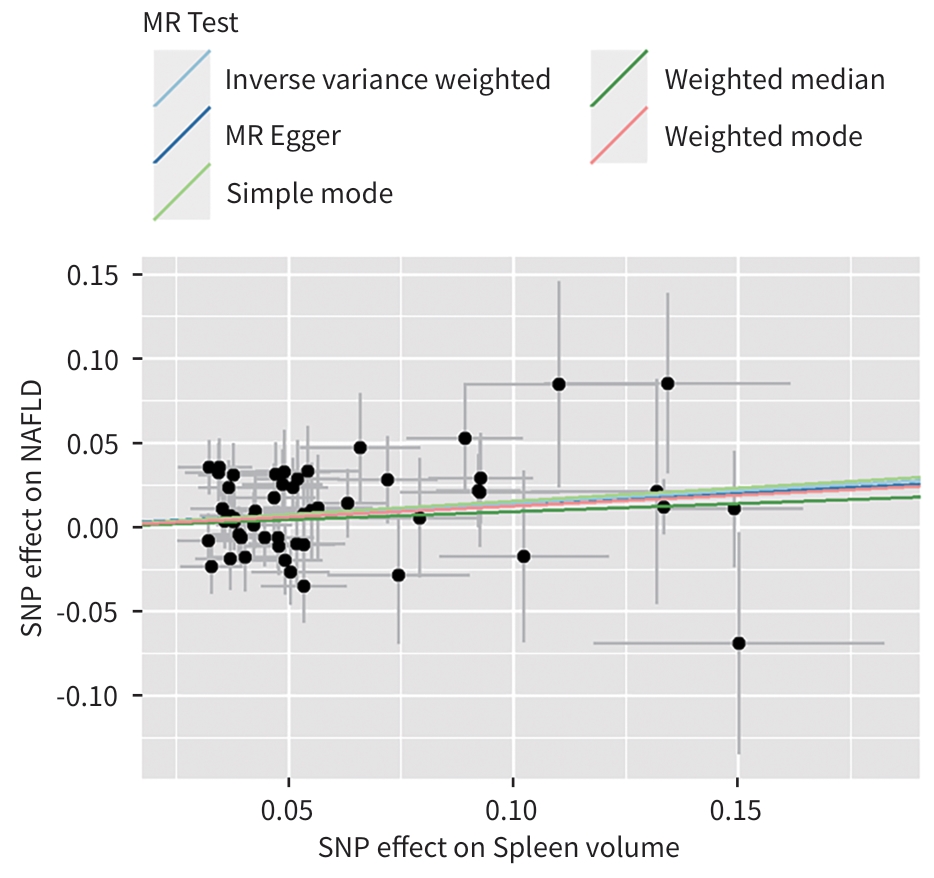
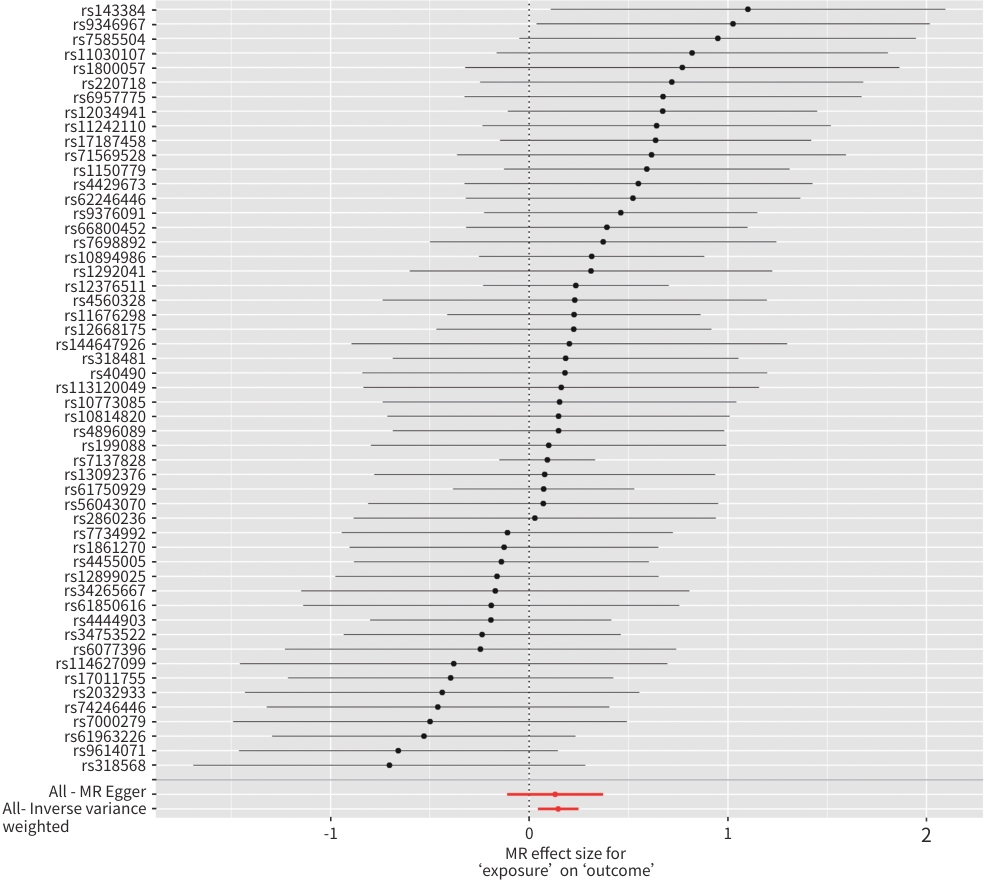
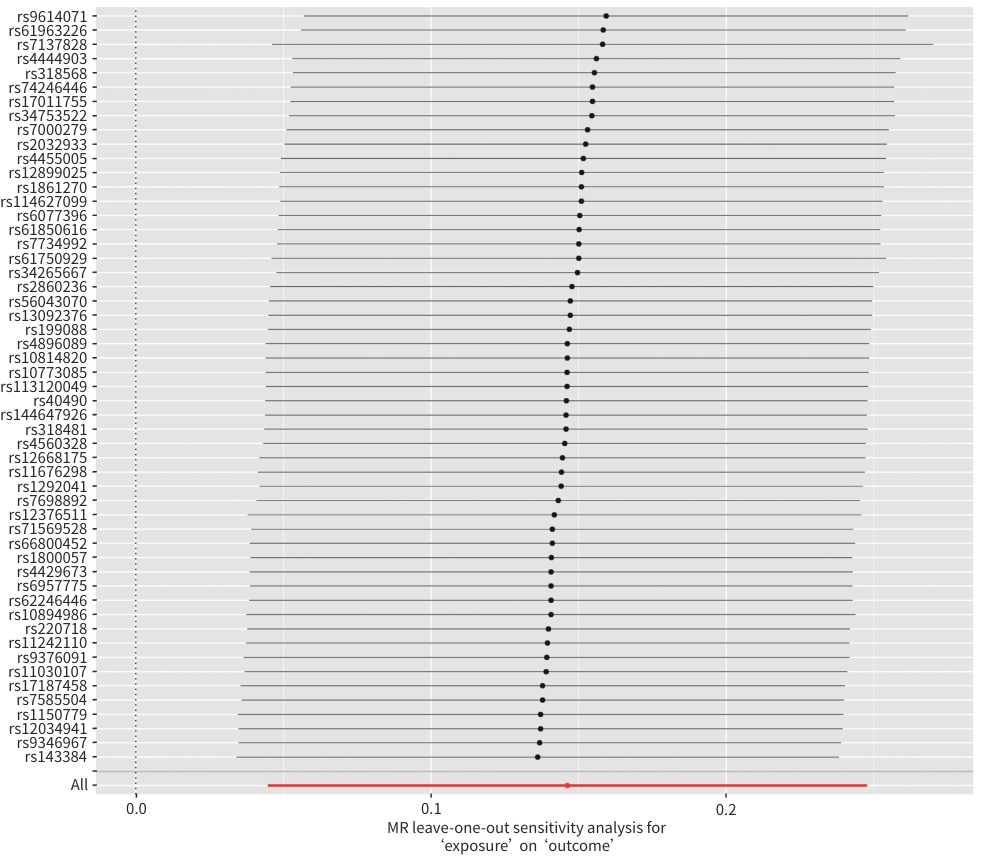
MR方法 SNP OR(95%CI) P值 加权模式 53 
1.14(0.94~1.38) 0.194 加权中位数法 53 1.10(0.93~1.30) 0.269 简单模式 53 1.17(0.85~1.61) 0.338 MR-Egger 53 1.14(0.90~1.45) 0.294 IVW 53 1.16(1.05~1.28) 0.005 注:暴露变量,脾脏体积;结局变量,NAFLD。
-
[1] GANGOPADHYAY A, IBRAHIM R, THEBERGE K, et al. Non-alcoholic fatty liver disease(NAFLD) and mental illness: Mechanisms linking mood, metabolism and medicines[J]. Front Neurosci, 2022, 16: 1042442. DOI: 10.3389/fnins.2022.1042442. [2] JICHITU A, BUNGAU S, STANESCU AMA, et al. Non-alcoholic fatty liver disease and cardiovascular comorbidities: Pathophysiological links, diagnosis, and therapeutic management[J]. Diagnostics(Basel), 2021, 11( 4): 689. DOI: 10.3390/diagnostics11040689. [3] THOMAS JA, KENDALL BJ, EL-SERAG HB, et al. Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease[J]. Lancet Gastroenterol Hepatol, 2024, 9( 2): 159- 169. DOI: 10.1016/S2468-1253(23)00275-3. [4] PENG HL, LIU LN, LIU DL, et al. Depression and non-alcoholic fatty liver disease: Association and potential mechanisms[J]. World Chin J Dig, 2022, 30( 7): 295- 302.彭海玲, 刘丽妮, 刘德良, 等. 抑郁症和非酒精性脂肪性肝病: 相关性及潜在机制[J]. 世界华人消化杂志, 2022, 30( 7): 295- 302. [5] TARANTINO G, CITRO V, BALSANO C. Liver-spleen axis in nonalcoholic fatty liver disease[J]. Expert Rev Gastroenterol Hepatol, 2021, 15( 7): 759- 769. DOI: 10.1080/17474124.2021.1914587. [6] TARGHER G, LONARDO A, BYRNE CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus[J]. Nat Rev Endocrinol, 2018, 14( 2): 99- 114. DOI: 10.1038/nrendo.2017.173. [7] LIU Y, BASTY N, WHITCHER B, et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning[J]. eLife, 2021, 10: e65554. DOI: 10.7554/eLife.65554. [8] KOGA Y, FUJITA M, NAKAHARA T, et al. Splenic volume in severe sepsis is associated with disease severity and pneumococcal infection[J]. Acute Med Surg, 2016, 3( 4): 339- 344. DOI: 10.1002/ams2.204. [9] KHOSHPOURI P, GHADIMI M, REZVANI HABIBABADI R, et al. Cross-sectional imaging in patients with primary sclerosing cholangitis: Single time-point liver or spleen volume is associated with survival[J]. Eur J Radiol, 2020, 132: 109331. DOI: 10.1016/j.ejrad.2020.109331. [10] BARREA L, DI SOMMA C, MUSCOGIURI G, et al. Nutrition, inflammation and liver-spleen axis[J]. Crit Rev Food Sci Nutr, 2018, 58( 18): 3141- 3158. DOI: 10.1080/10408398.2017.1353479. [11] TARANTINO G, SCALERA A, FINELLI C. Liver-spleen axis: Intersection between immunity, infections and metabolism[J]. World J Gastroenterol, 2013, 19( 23): 3534- 3542. DOI: 10.3748/wjg.v19.i23.3534. [12] ZHANG SY, WAN D, ZHU MC, et al. CD11b+CD43hi Ly6Clo splenocyte-derived macrophages exacerbate liver fibrosis via spleen-liver axis[J]. Hepatology, 2023, 77( 5): 1612- 1629. DOI: 10.1002/hep.32782. [13] ZHANG X, LEI B, YUAN Y, et al. Brain control of humoral immune responses amenable to behavioural modulation[J]. Nature, 2020, 581( 7807): 204- 208. DOI: 10.1038/s41586-020-2235-7. [14] QIN XY, CHEN DF, HU YH. Application of Mendeiian randomization in the etioiogicai study[J]. Chin J Epidemiol, 2006, 27( 7): 630- 633. DOI: 10.3760/j.issn: 0254-6450.2006.07.020.秦雪英, 陈大方, 胡永华. 孟德尔随机化方法在流行病学病因推断中的应用[J]. 中华流行病学杂志, 2006, 27( 7): 630- 633. DOI: 10.3760/j.issn:0254-6450.2006.07.020. [15] NI JJ, XU Q, YAN SS, et al. Gut microbiota and psychiatric disorders: A two-sample mendelian randomization study[J]. Front Microbiol, 2022, 12: 737197. DOI: 10.3389/fmicb.2021.737197. [16] LONG YW, TANG LH, ZHOU YY, et al. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study[J]. BMC Med, 2023, 21( 1): 66. DOI: 10.1186/s12916-023-02761-6. [17] LIANG H, MU HB, ZHANG FH, et al. Causal relationship between linoleic acid and type 2 diabetes and glycemic traits: A bidirectional Mendelian randomization study[J]. Front Endocrinol(Lausanne), 2023, 14: 1277153. DOI: 10.3389/fendo.2023.1277153. [18] Fatty Liver and Alcoholic Liver Disease Group of the Hepatology Branch of the Chinese Medical Association, Steatotic Liver Disease Expert Committee of the Chinese Physicians Association. Guide lines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001- 5256.2018.05.007 [19] ÇIÇEK Ö, ABDULKADIR A, LIENKAMP SS, et al. 3D U-Net: Learning dense volumetric segmentation from sparse annotation[C]// Medical Image Computing and Computer-Assisted Intervention-MICCAI 2016. Cham: Springer International Publishing, 2016: 424- 432. DOI: 10.1007/978-3-319-46723-8_49. [20] LI JJ, ZHAO SP, ZHAO D, et al. 2023 Chinese guideline for lipid management[J]. Front Pharmacol, 2023, 14: 1190934. DOI: 10.3389/fphar.2023.1190934. [21] LEE JH, KIM D, KIM HJ, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease[J]. Dig Liver Dis, 2010, 42( 7): 503- 508. DOI: 10.1016/j.dld.2009.08.002. [22] GHODSIAN N, ABNER E, EMDIN CA, et al. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease[J]. Cell Rep Med, 2021, 2( 11): 100437. DOI: 10.1016/j.xcrm.2021.100437. [23] POLIMENI L, PASTORI D, BARATTA F, et al. Spleen dimensions are inversely associated with lysosomal acid lipase activity in patients with non-alcoholic fatty liver disease[J]. Intern Emerg Med, 2017, 12( 8): 1159- 1165. DOI: 10.1007/s11739-017-1746-1. [24] SUZUKI K, KIRIKOSHI H, YONEDA M, et al. Measurement of spleen volume is useful for distinguishing between simple steatosis and early-stage non-alcoholic steatohepatitis[J]. Hepatol Res, 2010, 40( 7): 693- 700. DOI: 10.1111/j.1872-034X.2010.00643.x. [25] TSUSHIMA Y, ENDO K. Spleen enlargement in patients with nonalcoholic fatty liver: Correlation between degree of fatty infiltration in liver and size of spleen[J]. Dig Dis Sci, 2000, 45( 1): 196- 200. DOI: 10.1023/a:1005446418589. [26] SHI YQ, GUO GY. Physiological connection between spleen and liver and their interactions in liver diseases[J]. Chin J Dig, 2018, 38( 2): 83- 86. DOI: 10.3760/cma.j.issn.0254-1432.2018.02.005.时永全, 郭冠亚. 肝脏与脾脏的生理联系及其在肝病中的相互作用[J]. 中华消化杂志, 2018, 38( 2): 83- 86. DOI: 10.3760/cma.j.issn.0254-1432.2018.02.005. [27] GAO WH, GE KX, XIANG XX. Association of Th17 cells, regulatory T cells, and their imbalance with nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2018, 34( 6): 1347- 1350. DOI: 10.3969/j.issn.1001-5256.2018.06.046.高伟华, 葛宽雪, 向晓星. 辅助性T淋巴细胞17和调节性T淋巴细胞及其平衡改变与非酒精性脂肪性肝病的关系[J]. 临床肝胆病杂志, 2018, 34( 6): 1347- 1350. DOI: 10.3969/j.issn.1001-5256.2018.06.046. [28] HE BH, WU LY, XIE W, et al. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice[J]. BMC Immunol, 2017, 18( 1): 33. DOI: 10.1186/s12865-017-0215-y. [29] TARANTINO G, CONCA P, PASANISI F, et al. Could inflammatory markers help diagnose nonalcoholic steatohepatitis?[J]. Eur J Gastroenterol Hepatol, 2009, 21( 5): 504- 511. DOI: 10.1097/MEG.0b013e3283229b40. [30] GU XC, MA ZY, FANG J, et al. Obesity enhances antioxidant capacity and reduces cytokine levels of the spleen in mice to resist splenic injury challenged by Escherichia coli[J]. J Immunol Res, 2020, 2020: 5948256. DOI: 10.1155/2020/5948256. [31] ZHAO P, JIN H, ZHU JX, et al. Role of interleukin-1β, interleukin-6 and tumor necrosis factor-α in the development of nonalcoholic fatty liver disease[J]. Hainan Med J, 2022, 33( 1): 96- 99.赵鹏, 金海, 朱加兴, 等. 白细胞介素-1β、白细胞介素-6与肿瘤坏死因子-α在非酒精性脂肪性肝病发展过程中的作用[J]. 海南医学, 2022, 33( 1): 96- 99. [32] LEITE ND, MONTES EG, FISHER SV, et al. Splenectomy attenuates obesity and decreases insulin hypersecretion in hypothalamic obese rats[J]. Metabolism, 2015, 64( 9): 1122- 1133. DOI: 10.1016/j.metabol.2015.05.003. [33] KERAMIDA G, DUNFORD A, KAYA G, et al. Hepato-splenic axis: Hepatic and splenic metabolic activities are linked[J]. Am J Nucl Med Mol Imaging, 2018, 8( 3): 228- 238. [34] WANG ZM, LI NS, WANG B, et al. Nonalcoholic fatty liver disease progression in rats is accelerated by splenic regulation of liver PTEN/AKT[J]. Saudi J Gastroenterol, 2015, 21( 4): 232- 238. DOI: 10.4103/1319-3767.161641. [35] LI NS, WANG ZM, LIN JH. Up-regulated expression of PTEN after splenetomy may prevent the progression of liver fibrosis in rats[J]. J Hepatobiliary Pancreat Sci, 2016, 23( 1): 50- 56. DOI: 10.1002/jhbp.300. [36] MUROTOMI K, TAWARA H, SUTOH M, et al. Iron-accumulating splenocytes may exacerbate non-alcoholic steatohepatitis through the production of proinflammatory cytokines and reactive oxygen species[J]. Exp Biol Med(Maywood), 2022, 247( 10): 848- 855. DOI: 10.1177/15353702221077218. [37] CACCIOTTOLO TM, KUMAR A, GODFREY EM, et al. Spleen size does not correlate with histological stage of liver disease in people with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2023, 21( 2): 535- 537.e1. DOI: 10.1016/j.cgh.2022.01.007. [38] HAMABE A, UTO H, IMAMURA Y, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period[J]. J Gastroenterol, 2011, 46( 6): 769- 778. DOI: 10.1007/s00535-011-0376-z. [39] CHEN JH, ZHOU H, JIN HW, et al. Role of inflammatory factors in mediating the effect of lipids on nonalcoholic fatty liver disease: A two-step, multivariable mendelian randomization study[J]. Nutrients, 2022, 14( 20): 4434. DOI: 10.3390/nu14204434. [40] HU YJ, LIU J, DONG XJ, et al. Clinical study of serum homocysteine and non-alcoholic fatty liver disease in euglycemic patients[J]. Med Sci Monit, 2016, 22: 4146- 4151. DOI: 10.12659/msm.897924. [41] CIGRI E, INAN FC, ER ER, et al. The relationship between lipid profile and non-alcoholic fatty liver disease in children and adolescents with obesity[J]. J Coll Physicians Surg Pak, 2022, 32( 5): 591- 595. DOI: 10.29271/jcpsp.2022.05.591. [42] QIU JJ, KUANG MB, YANG RJ, et al. The newly proposed alanine aminotransferase to high-density lipoprotein cholesterol ratio has shown effectiveness in identifying non-alcoholic fatty liver disease[J]. Front Endocrinol(Lausanne), 2023, 14: 1239398. DOI: 10.3389/fendo.2023.1239398. [43] SUN DQ, LIU WY, WU SJ, et al. Increased levels of low-density lipoprotein cholesterol within the normal range as a risk factor for nonalcoholic fatty liver disease[J]. Oncotarget, 2016, 7( 5): 5728- 5737. DOI: 10.18632/oncotarget.6799. [44] WANG S, LIN XH, ZHU CC, et al. Association between nonalcoholic fatty liver disease and increased glucose-to-albumin ratio in adults without diabetes[J]. Front Endocrinol(Lausanne), 2024, 14: 1287916. DOI: 10.3389/fendo.2023.1287916. [45] CHOI SH, OH DJ, KWON KH, et al. A vegetarian diet does not protect against nonalcoholic fatty liver disease(NAFLD): A cross-sectional study between Buddhist priests and the general population[J]. Turk J Gastroenterol, 2015, 26( 4): 336- 343. DOI: 10.5152/tjg.2015.0046. [46] ZHANG M, CHEN L, ZHAI MD, et al. Analysis of the risk factors for NAFLD in patients with T2DM[J]. Parenter Enter Nutr, 2019, 26( 5): 271- 275. DOI: 10.16151/j.1007-810x.2019.05.004.张敏, 陈璐, 瞿美娣, 等. 2型糖尿病病人合并非酒精性脂肪性肝病的危险因素分析[J]. 肠外与肠内营养, 2019, 26( 5): 271- 275. DOI: 10.16151/j.1007-810x.2019.05.004. [47] HAN XC, XU PF, ZHOU JM, et al. Fasting C-peptide is a significant indicator of nonalcoholic fatty liver disease in obese children[J]. Diabetes Res Clin Pract, 2020, 160: 108027. DOI: 10.1016/j.diabres.2020.108027. [48] YIN SM, ZHENG SB, ZHOU H, et al. Age-related changes in serum albumin, globulin and hemoglobin levels in healthy normal populations[J]. Chin J Gerontol, 2010, 30( 9): 1201- 1203.尹曙明, 郑松柏, 周骅, 等. 健康正常人群血清白蛋白、球蛋白、血红蛋白水平的增龄变化[J]. 中国老年学杂志, 2010, 30( 9): 1201- 1203. -



 PDF下载 ( 9727 KB)
PDF下载 ( 9727 KB)


 下载:
下载: