慢性丙型肝炎基因3型患者的临床特征: 一项多中心回顾性队列研究
DOI: 10.12449/JCH250811
Clinical features of chronic hepatitis C patients with genotype 3 infection: A multicenter retrospective cohort study
-
摘要:
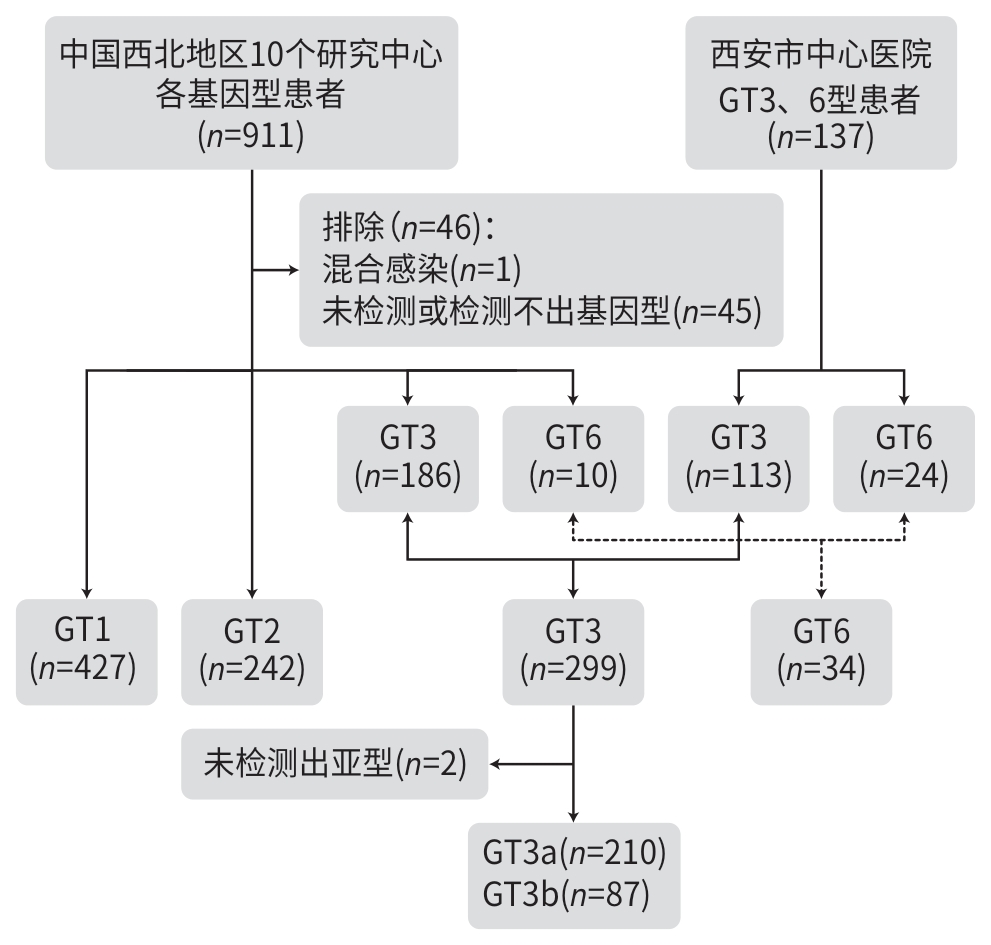
目的 探讨慢性丙型肝炎(CHC)基因3型(GT3)感染者的人群特征及疾病进展相关危险因素。 方法 本研究为一项多中心回顾性队列研究,纳入2017年12月—2023年11月中国西北地区11个临床中心的1 002例CHC患者,根据CHC GT类型将患者分为GT1、GT2、GT3及GT6组,比较分析不同基因型患者的临床特征。符合正态分布的计量资料组间比较采用单因素方差分析,进一步两两比较采用Scheffe法;不符合正态分布的计量资料组间比较采用Kruskal-Wallis H检验。计数资料组间比较采用χ2检验或Fisher精确检验。采用多因素Logistic回归分析评估CHC进展为肝硬化的相关影响因素。 结果 纳入CHC患者GT1 427例、GT2 242例、GT3 299例(GT3a 210例,GT3b 87例,未分亚型2例)、GT6 34例。GT3患者年龄(51.3±0.5)岁,明显低于GT1[(53.2±0.6)岁]患者及GT2[(53.7±0.8)岁]患者(P值均<0.05),尤其是肝硬化患者中,GT3年龄[(52.1±0.5)岁]明显低于GT1[(59.4±0.9)岁]、GT2[(58.1±1.1)岁](P值均<0.05);GT3男性比例和肝硬化比例分别为77.9%和46.2%,均高于GT1、GT2和GT6患者(P值均<0.05)。GT3患者基线ALT、AST水平明显高于GT1及GT2患者,APRI指数和FIB-4指数均显著高于GT1、GT2和GT6患者,PLT计数明显低于GT2和GT6患者,AFP水平明显高于GT2和GT6患者,Alb水平明显低于GT6患者(P值均<0.05)。GT3a与GT3b亚型患者的年龄、性别、肝硬化比例、合并症、HCV RNA定量、PLT、ALT、AST、ALP、Alb、AST/PLT比值指数(APRI)及纤维化-4指数(FIB-4)等均无统计学差异(P值均>0.05)。多因素Logistic回归分析显示,PLT≤150×109/L(OR=10.72,95%CI:5.76~35.86,P<0.001)和Alb≤35 g/L(OR=3.74,95%CI:1.22~11.45,P=0.021)是GT3型患者发生肝硬化的独立危险因素。 结论 中国西北地区CHC GT3患者以男性为主;与其他基因型相比,发病年龄较轻,肝脏炎症活动度和纤维化程度更为显著;低PLT计数和低白蛋白水平为CHC GT3患者进展为肝硬化的危险因素。 Abstract:Objective To investigate the clinical features of chronic hepatitis C (CHC) patients with hepatitis C virus genotype 3 (HCV GT3) infection and the risk factors for disease progression. Methods A multicenter retrospective cohort study was conducted among 1 002 CHC patients from 11 clinical centers in Northwest China from December 2017 to November 2023, and according to their genotype, they were divided into GT1, GT2, GT3, and GT6 groups. Clinical features were compared between the patients with different genotypes. The one-way analysis of variance was used for comparison of normally distributed continuous data between groups, and the Scheffe test was used for further comparison between two groups. The Kruskal-Wallis H test was used for comparison of data with skewed distribution between groups; the chi-square test or Fisher test was used for comparison of categorical data between groups. The multivariate logistic regression analysis was used to explore the influencing factors for the progression of CHC to liver cirrhosis. Results In terms of the genotype, there were 427 patients with GT1 infection, 242 with GT2 infection, 299 with GT3 infection (210 patients with GT3a infection, 87 with GT3b infection, and 2 with unclassified genotype), and 34 with GT6 infection. The patients with GT3 infection had a significantly younger age than those with GT1 infection (51.3±0.5 years vs 53.2±0.6 years, P<0.05) or GT2 infection (51.3±0.5 years vs 53.7±0.8 years, P<0.05), and for the patients with liver cirrhosis, the patients with GT3 infection had a significantly younger age than those with GT1 infection (52.1±0.5 years vs 59.4±0.9 years, P<0.001) or GT2 infection (52.1±0.5 years vs 58.1±1.1 years, P<0.001). Among the patients with GT3 infection, male patients accounted for 77.9% and the patients with liver cirrhosis accounted for 46.2%, which were significantly higher than those among the patients with GT1, GT2 or GT6 infection (all P<0.001). At baseline, the patients with GT3 infection had significantly higher levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) than those with GT1 or GT2 infection, significantly higher aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 (FIB4) than those with GT1, GT2 or GT6 infection, a significantly lower platelet count (PLT) than those with GT2 or GT6 infection, a significantly higher level of alpha-fetoprotein than those with GT2 or GT6 infection, and a significantly lower level of albumin (Alb) than those with GT6 infection (all P<0.05). There were no significant differences between the patients with GT3a infection and those with GT3b infection in age, sex, the proportion of patients with liver cirrhosis, comorbidities, HCV RNA quantification, PLT, ALT, AST, alkaline phosphatase, Alb, APRI, and FIB-4 (all P>0.05). The multivariate logistic regression analysis showed that PLT≤150×109/L (odds ratio [OR]=10.72, 95% confidence interval [CI]: 5.76 — 35.86, P<0.001) and Alb≤35 g/L (OR=3.74, 95%CI: 1.22 — 11.45, P=0.021) were risk factors for liver cirrhosis. Conclusion Most CHC patients with GT3 infection are male in Northwest China, and compared with the patients with other genotypes, such patients tend to have a younger age of onset and higher degrees of liver inflammation activity and fibrosis. Low PLT and a low level of Alb are risk factors for progression to liver cirrhosis in CHC patients with GT3 infection. -
Key words:
- Hepatitis C, Chronic /
- Epidemiology /
- Liver Cirrhosis /
- Risk factors
-
表 1 HCV不同基因型患者基线临床特征
Table 1. Baseline clinical characteristics of patients infected with different HCV genotype
指标 GT1(n=427) GT2(n=242) GT3(n=299) GT6(n=34) 统计值 P值 年龄(岁) 53.2±0.61) 53.7±0.81) 51.3±0.5 50.0±1.4 F=2.937 0.032 男性[例(%)] 251(58.8)1) 125(51.7)1) 233(77.9) 12(35.3)1) χ²=55.343 <0.001 肝硬化[例(%)] 167(39.1)1) 88(36.4)1) 138(46.2) 5(14.7)1) χ²=17.624 <0.001 年龄(岁) 59.4±0.91) 58.1±1.11) 52.1±0.5 55.8±2.51) F=14.589 <0.001 肝细胞癌[例(%)] 10(2.3) 5(2.1) 10(3.3) 1(2.9) 0.775 合并症[例(%)] 肾功能不全a 14(3.4) 13(5.4) 9(3.0) 0(0.0) 0.322 糖尿病b 10(6.7) 10(11.2) 29(9.7) 2(6.5) 0.588 高血压c 15(10.2) 6(6.8) 39(13.0) 2(6.5) 0.307 脑血管病d 4(2.7) 1(1.1) 12(4.0) 2(6.5) 0.422 既往治疗史e[例(%)] 0.253 无 408(96.0) 229(95.4) 277(93.6) 34(100.0) 干扰素经治 5(1.2) 8(3.3) 13(4.4) 0(0.0) DAA经治 12(2.8) 3(1.3) 6(2.0) 0(0.0) 注:与GT3比较,1)P<0.05。a,肾功能不全数据缺失:GT1组缺失19例,GT2组缺失2例;b,糖尿病数据缺失:GT1组缺失278例,GT2组缺失153例,GT6组缺失3例;c,高血压数据缺失:GT1组缺失280例,GT2组缺失154例,GT6组缺失3例;d,脑血管病数据缺失:GT1组缺失280例,GT2组缺失154例,GT6组缺失3例;e,既往治疗史数据缺失:GT1组缺失2例,GT2组缺失2例,GT3组缺失3例。
表 2 HCV不同基因型患者基线实验室检查特征
Table 2. Baseline laboratory results of patients infected with different HCV genotype
指标 GT1(n=427) GT2(n=242) GT3(n=299) GT6(n=34) 统计值 P值 HCV RNAa(log10 IU/mL) 6.34±0.97 6.26±1.06 6.26±1.14 6.44±1.22 F=2.128 0.536 WBC(×109/L) 4.86(3.89~6.35) 4.97(3.93~6.33) 5.10(3.87~6.40) 5.49(4.59~6.96) H=3.116 0.374 PLT(×109/L) 139.0(95.0~198.0) 150.5(97.8~203.5)1) 130.0(75.5~185.0) 174.0(143.0~226.0)1) H=18.544 0.002 Hb(g/dL) 138.0(127.0~152.0)1) 140.0(127.5~153.0)1) 158.0(133.0~160.0) 154.0(131.0~166.0) H=244.698 0.001 AST(U/L) 54.62±1.901) 59.24±3.801) 79.02±3.08 59.78±10.411) F=15.507 <0.001 ALT(U/L) 60.95±2.621) 69.99±5.281) 90.1±4.21 76.94±10.42 F=10.982 <0.001 ALP(U/L) 100.41±2.28 101.33±5.36 107.68±3.25 99.4±7.78 F=0.962 0.410 Albb(g/L) 41.44±0.33 41.27±0.45 40.61±0.41 43.98±0.861) F=2.837 0.037 AFPc(ng/mL) 4.75(2.95~8.11) 4.46(2.59~8.06)1) 5.34(3.23~11.63) 2.85(1.91~5.14)1) H=16.489 <0.001 血清肌酐d(μmol/L) 67.63±4.29 68.40±4.88 64.21±1.53 63.80±2.35 F=0.241 0.868 APRI指数e 0.86(0.42~1.62)1) 0.69(0.38~1.49)1) 1.40(0.74~2.41) 0.57(0.32~1.14)1) H=58.24 <0.001 FIB-4指数f 2.65(1.38~4.59)1) 2.31(1.29~4.80)1) 3.13(1.91~6.07) 1.69(1.22~2.68)1) H=26.989 <0.001 注:与GT3比较,1)P<0.05。a,HCV RNA数据缺失:GT1组缺失25例,GT2组缺失17例,GT3组缺失13例,GT6组缺失1例;b,Alb数据缺失:GT1组缺失32例,GT2组缺失22例,GT3组缺失19例,GT6组缺失2例;c,AFP数据缺失:GT1组缺失139例,GT2组缺失55例,GT3组缺失133例,GT6组缺失16例;d,血清肌酐数据缺失:GT1组缺失75例,GT2组缺失55例,GT3组缺失95例,GT6组缺失10例;e,APRI指数数据缺失:GT1组缺失53例,GT2组缺失24例,GT3组缺失35例,GT6组缺失4例;f,FIB-4指数数据缺失:GT1组缺失53例,GT2组缺失25例,GT3组缺失35例,GT6组缺失4例。
表 3 HCV基因3a/3b亚型患者基线临床特征
Table 3. Baseline characteristics of HCV genotype 3a/3b subtypes infections
指标 GT3a(n=210) GT3b(n=87) 统计值 P值 年龄(岁) 51.44±0.60 50.8±0.82 t=0.357 0.551 男性[例(%)] 159(75.7) 68(78.2) χ²=0.015 0.347 肝硬化[例(%)] 99(47.1) 38(43.7) χ²=0.522 0.296 年龄(岁) 52.05±0.86 52.58±1.15 t=0.135 0.736 肝细胞癌a[例(%)] 10(4.8) 0(0.0) 0.028 合并症[例(%)] 肾功能不全 7(3.3) 1(1.1) 0.342 糖尿病 18(8.6) 10(11.5) 0.280 高血压 26(12.4) 13(14.9) 0.337 脑血管病 8(3.8) 4(4.6) 0.487 既往治疗史[例(%)] 无 200(95.2) 77(88.5) 干扰素经治 9(4.3) 5(5.7) DAA经治 1(0.5) 5(5.7) HCV RNAb(log10 IU/mL) 6.22±1.43 6.33±0.99 t=0.571 0.466 WBC(×109/L) 4.95(3.84~6.12) 5.46(4.14~6.77) H=4.424 0.035 PLT(×109/L) 126.5(76~176.25) 140.5(84.25~191.25) H=0.710 0.392 AFPc(ng/mL) 5.18(3.36~11.52) 6.14(3.17~13.03) H=0.124 0.724 AST(U/L) 77.93±3.52 82.19±5.99 t=0.394 0.525 ALT(U/L) 88.74±4.28 94.28±8.09 t=0.365 0.545 ALP(U/L) 107.64±3.97 106.85±5.61 t=0.016 0.913 Alb(g/L) 40.43±0.50 41.31±0.69 t=1.053 0.328 血清肌酐d(μmol/L) 64.89±1.96 62.86±2.25 t=0.354 0.553 注:a,肝细胞癌数据缺失:GT3a组缺失3例;b,HCV RNA数据缺失:GT3a组缺失10例,GT3b组缺失3例;c,AFP数据缺失:GT3a组缺失100例,GT3b组缺失32例;d,血清肌酐数据缺失:GT3a组缺失65例,GT3b组缺失29例。
表 4 基因3型患者肝硬化相关危险因素的单因素和多因素Logistic分析
Table 4. Univariate and multivariate logistic analysis of risk factors for cirrhosis in patients with genotype 3 HCV infection
项目 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 性别(男/女) 1.48(1.14~1.93) 0.205 年龄(>50/≤50岁) 1.14(0.70~1.86) 0.608 基因亚型(GT3a/非GT3a) 0.83(0.50~1.38) 0.470 酒精(是/否) 2.06(0.87~4.89) 0.102 吸烟(是/否) 1.97(1.18~3.28) 0.009 合并肾病(是/否) 0.84(0.19~3.84) 0.827 合并糖尿病(是/否) 1.98(0.90~4.35) 0.090 合并高血压(是/否) 1.30(0.66~2.57) 0.452 合并脑血管病(是/否) 0.80(0.25~2.58) 0.709 既往治疗史 无治疗(是/否) 3.37(1.38~8.04) 0.013 干扰素经治(是/否) 6.81(1.48~31.29) 0.014 DAA经治(是/否) 6.19(0.71~53.68) 0.098 HCV RNA(>107/≤107 IU/mL) 0.43(0.25~0.75) 0.003 PLT(≤150/>150×109/L) 12.53(6.72~23.38) <0.001 10.72(5.76~35.86) <0.001 WBC(>LLN/≤LLN) 5.71(3.12~10.45) <0.001 AST(>1.5/≤1.5×ULN) 0.99(0.63~1.57) 0.974 ALT(>1.5/≤1.5×ULN) 0.58(0.37~0.93) 0.023 Alb(≤35/>35 g/L) 7.81(3.64~16.77) <0.001 3.74(1.22~11.45) 0.021 AFP(≤7/>7 ng/mL) 2.16(1.14~4.10) 0.019 -
[1] Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study[J]. Lancet Gastroenterol Hepatol, 2022, 7( 5): 396- 415. DOI: 10.1016/S2468-1253(21)00472-6. [2] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infections Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045. [3] Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study[J]. Lancet Gastroenterol Hepatol, 2017, 2( 3): 161- 176. DOI: 10.1016/S2468-1253(16)30181-9. [4] MESSINA JP, HUMPHREYS I, FLAXMAN A, et al. Global distribution and prevalence of hepatitis C virus genotypes[J]. Hepatology, 2015, 61( 1): 77- 87. DOI: 10.1002/hep.27259. [5] RAO HY, WEI L, LOPEZ-TALAVERA JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection[J]. J Gastroenterol Hepatol, 2014, 29( 3): 545- 553. DOI: 10.1111/jgh.12398. [6] TANG Q, CHEN ZW, LI H, et al. Molecular epidemiology of hepatitis C virus genotypes in different geographical regions of Chinese mainland and a phylogenetic analysis[J]. Infect Dis Poverty, 2023, 12( 1): 66. DOI: 10.1186/s40249-023-01106-y. [7] CHEN Y, YU CS, YIN XR, et al. Hepatitis C virus genotypes and subtypes circulating in Mainland China[J]. Emerg Microbes Infect, 2017, 6( 11): e95. DOI: 10.1038/emi.2017.77. [8] LI F, LI B, ZHU QY. Research progress on the epidemiological characteristics and diagnosis of hepatitis C in China[J]. Int J Virol, 2023, 30( 6): 509- 511. DOI: 10.3760/cma.j.issn.1673-4092.2023.06.014.黎锋, 李博, 朱秋映. 中国丙肝流行特征与诊断研究进展[J]. 国际病毒学杂志, 2023, 30( 6): 509- 511. DOI: 10.3760/cma.j.issn.1673-4092.2023.06.014. [9] BOJANIC K, BOGOJEVIC MS, VUKADIN S, et al. Noninvasive fibrosis assessment in chronic hepatitis C infection: An update[J]. J Clin Transl Hepatol, 2023, 11( 5): 1228- 1238. DOI: 10.14218/JCTH.2022.00365. [10] JI FP, LI J, LIU L, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b[J]. J Gastroenterol Hepatol, 2021, 36( 3): 767- 774. DOI: 10.1111/jgh.15192. [11] HUANG CF, IIO E, JUN DW, et al. Direct-acting antivirals in East Asian hepatitis C patients: Real-world experience from the REAL-C Consortium[J]. Hepatol Int, 2019, 13( 5): 587- 598. DOI: 10.1007/s12072-019-09974-z. [12] HUANG R, RAO HY, XIE Q, et al. Comparison of the efficacy of sofosbuvir plus ribavirin in Chinese patients with genotype 3a or 3b HCV infection[J]. J Med Virol, 2019, 91( 7): 1313- 1318. DOI: 10.1002/jmv.25454. [13] WU N, RAO HY, YANG WB, et al. Impact of hepatitis C virus genotype 3 on liver disease progression in a Chinese national cohort[J]. Chin Med J(Engl), 2020, 133( 3): 253- 261. DOI: 10.1097/CM9.0000000000000629. [14] DEGENHARDT L, PEACOCK A, COLLEDGE S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review[J]. Lancet Glob Health, 2017, 5( 12): e1192- e1207. DOI: 10.1016/S2214-109X(17)30375-3. [15] ADINOLFI LE, UTILI R, ANDREANA A, et al. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C[J]. Dig Dis Sci, 2001, 46( 8): 1677- 1683. DOI: 10.1023/a:1010697319589. [16] LIU CR, LI X, CHAN PL, et al. Prevalence of hepatitis C virus infection among key populations in China: A systematic review[J]. Int J Infect Dis, 2019, 80: 16- 27. DOI: 10.1016/j.ijid.2018.11.006. [17] HAQUE LY, FIELLIN DA, TATE JP, et al. Association between alcohol use disorder and receipt of direct-acting antiviral hepatitis C virus treatment[J]. JAMA Netw Open, 2022, 5( 12): e2246604. DOI: 10.1001/jamanetworkopen.2022.46604. [18] IM PK, MILLWOOD IY, KARTSONAKI C, et al. Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: A 10-year prospective study of 0.5 million adults[J]. BMC Med, 2021, 19( 1): 216. DOI: 10.1186/s12916-021-02079-1. [19] PREMKUMAR M, ANAND AC. Tobacco, cigarettes, and the liver: The smoking Gun[J]. J Clin Exp Hepatol, 2021, 11( 6): 700- 712. DOI: 10.1016/j.jceh.2021.07.016. [20] WEI L, LIM SG, XIE Q, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: A single-arm, open-label, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2019, 4( 2): 127- 134. DOI: 10.1016/S2468-1253(18)30343-1. [21] JI FP, TRAN S, OGAWA E, et al. Real-world effectiveness and tolerability of interferon-free direct-acting antiviral for 15, 849 patients with chronic hepatitis C: A multinational cohort study[J]. J Clin Transl Hepatol, 2024, 12( 7): 646- 658. DOI: 10.14218/JCTH.2024.00089. [22] ABULITIFU YLHM, LIAN JS, BALI RML, et al. Efficacy and safety of Sofosbuvir/Velpatasvir or combined with Ribavirin in treatment of patients with hepatitis C related cirrhosis in Xinjiang region[J]. Chin J Clin Infect Dis, 2022, 15( 6): 459- 463. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.005.依力哈木·阿不力提甫, 连江山, 热米拉·巴力, 等. 索磷布韦/维帕他韦单用或联合利巴韦林治疗新疆地区丙型肝炎肝硬化患者的疗效及安全性分析[J]. 中华临床感染病杂志, 2022, 15( 6): 459- 463. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.005. [23] LIU YY, LIU N, FU JJ, et al. Distribution characteristics of hepatitis C virus genotypes in Shaanxi Province[J]. Hainan Med J, 2021, 32( 4): 413- 416. DOI: 10.3969/j.issn.1003-6350.2021.04.002.刘媛媛, 刘娜, 付建军, 等. 陕西省丙型肝炎病毒基因型分布特征[J]. 海南医学, 2021, 32( 4): 413- 416. DOI: 10.3969/j.issn.1003-6350.2021.04.002. [24] ZHANG HY, RAO HY, CHEN HS. Current status of hepatitis C virus infection and progress in its elimination in China[J]. J Clin Hepatol, 2024, 40( 4): 649- 653. DOI: 10.12449/JCH240401.张海莹, 饶慧瑛, 陈红松. 中国丙型肝炎病毒感染的现状及清除进程[J]. 临床肝胆病杂志, 2024, 40( 4): 649- 653. DOI: 10.12449/JCH240401. -



 PDF下载 ( 967 KB)
PDF下载 ( 967 KB)

 下载:
下载:


