胰岛素清除在代谢相关脂肪性肝病中的作用
DOI: 10.12449/JCH250728
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:朱晓静负责论文框架设计及撰写;汤海林负责文献收集与论文修改;周梁负责拟定写作思路及图片制作;石俊负责指导论文撰写。
-
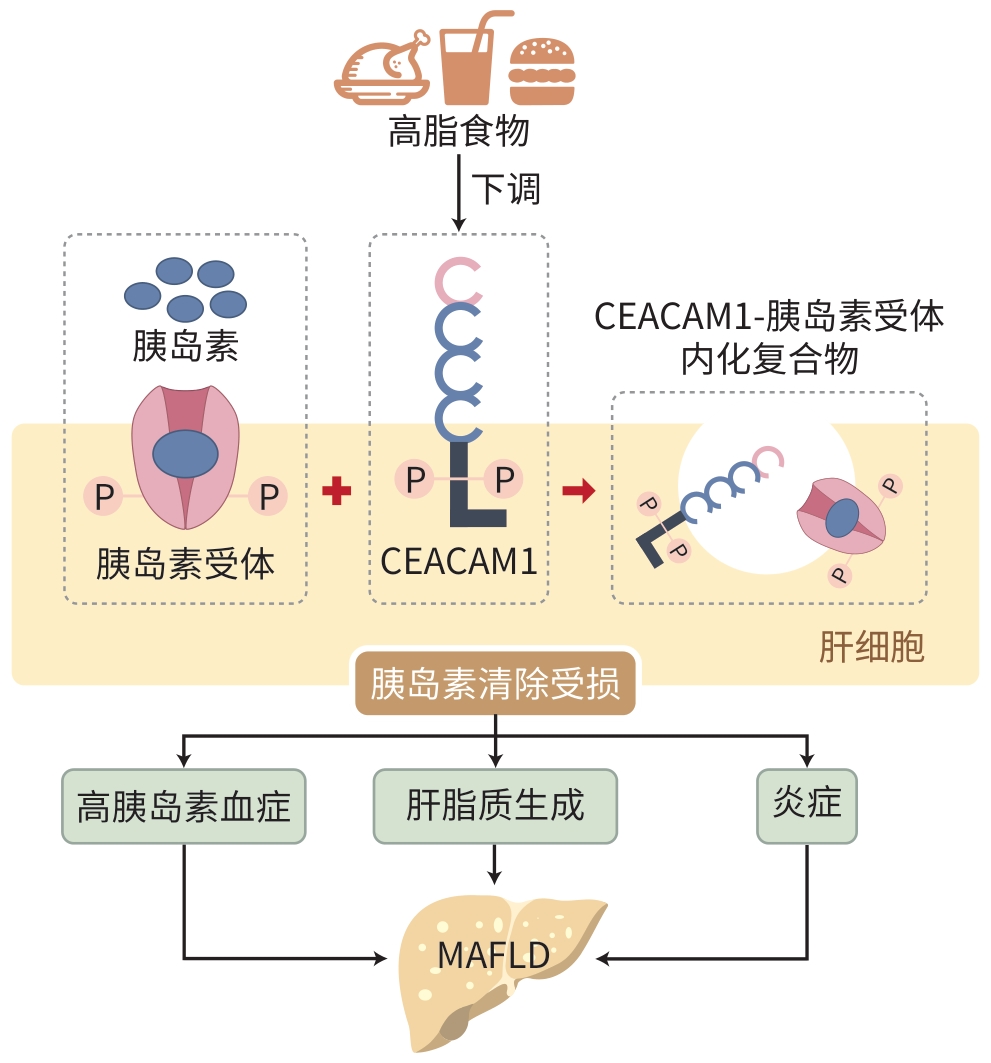
摘要: 随着生活方式的快速转变,我国非酒精性脂肪性肝病的患病趋势日益严峻,已成为一项重大的公共卫生问题。随着对该疾病认识的不断深入,最新的共识声明将非酒精性脂肪性肝病更名为代谢相关脂肪性肝病(MAFLD),其定义从原有的排除性诊断转变为包含性诊断。这一调整不仅提高了临床诊断效率,还进一步凸显了代谢紊乱在MAFLD进展中的关键作用。近年来,随着对癌胚抗原相关细胞黏附分子1介导的胰岛素清除机制的深入研究,胰岛素清除在MAFLD发生与发展中的重要性愈发显现。本文综述了胰岛素清除在MAFLD中的研究进展。
-
关键词:
- 代谢相关脂肪性肝病 /
- 非酒精性脂肪性肝病 /
- 胰岛素清除 /
- 癌胚抗原相关细胞黏附分子1
Abstract: With the rapid changes in lifestyle, the prevalence of nonalcoholic fatty liver disease (NAFLD) has become increasingly severe in China, and it has become a major public health concern. With a deeper understanding of this disease, the latest consensus statement has changed the name from NAFLD to MAFLD, and this updated definition transitions from an exclusion-based approach to an inclusive framework, which not only improves clinical diagnostic accuracy, but also highlights the key role of metabolic disorders in the progression of NAFLD. In recent years, the in-depth studies on the mechanism of carcinoembryonic antigen-associated cell adhesion molecule 1-mediated insulin clearance have highlighted the importance of insulin clearance in the development and progression of NAFLD. This article reviews the research advances in the role of insulin clearance in MAFLD. -
[1] PAN YQ, MAO AJ, YU CC, et al. Active components of traditional Chinese medicine and their compound prescriptions in prevention and treatment of nonalcoholic fatty liver disease: Current status and prospects[J]. J Clin Hepatol, 2024, 40( 10): 1933- 1941. DOI: 10.12449/JCH241002.潘雨晴, 毛傲洁, 于楚楚, 等. 中药有效成分及其复方防治非酒精性脂肪性肝病的现状与展望[J]. 临床肝胆病杂志, 2024, 40( 10): 1933- 1941. DOI: 10.12449/JCH241002. [2] LE MH, YEO YH, ZOU BY, et al. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical Bayesian approach[J]. Clin Mol Hepatol, 2022, 28( 4): 841- 850. DOI: 10.3350/cmh.2022.0239. [3] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158( 7): 1999- 2014. e 1. DOI: 10.1053/j.gastro.2019.11.312. [4] ESLAM M, NEWSOME PN, SARIN SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement[J]. J Hepatol, 2020, 73( 1): 202- 209. DOI: 10.1016/j.jhep.2020.03.039. [5] TENG TS, QIU S, ZHAO YM, et al. Pathogenesis and therapeutic strategies related to non-alcoholic fatty liver disease[J]. Int J Mol Sci, 2022, 23( 14): 7841. DOI: 10.3390/ijms23147841. [6] NAJJAR SM, CAPRIO S, GASTALDELLI A. Insulin clearance in health and disease[J]. Annu Rev Physiol, 2023, 85: 363- 381. DOI: 10.1146/annurev-physiol-031622-043133. [7] HE KOH, CAO C, MITTENDORFER B. Insulin clearance in obesity and type 2 diabetes[J]. Int J Mol Sci, 2022, 23( 2): 596. DOI: 10.3390/ijms23020596. [8] HAMMERMAN MR. Interaction of insulin with the renal proximal tubular cell[J]. Am J Physiol, 1985, 249( 1 Pt 2): F1- F11. DOI: 10.1152/ajprenal.1985.249.1.F1. [9] KUBE-GOLOVIN I, LYNDIN M, WIESEHÖFER M, et al. CEACAM expression in an in-vitro prostatitis model[J]. Front Immunol, 2023, 14: 1236343. DOI: 10.3389/fimmu.2023.1236343. [10] LEE WH, NAJJAR SM, KAHN CR, et al. Hepatic insulin receptor: New views on the mechanisms of liver disease[J]. Metabolism, 2023, 145: 155607. DOI: 10.1016/j.metabol.2023.155607. [11] XIN SL, XU KS. A new understanding of the pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2017, 33( 8): 1581- 1583. DOI: 10.3969/j.issn.1001-5256.2017.08.037.辛晟梁, 徐可树. 非酒精性脂肪性肝病发病机制新认识[J]. 临床肝胆病杂志, 2017, 33( 8): 1581- 1583. DOI: 10.3969/j.issn.1001-5256.2017.08.037. [12] DONG KX, CHEN DN, ZHENG Y, et al. The role of CEACAM1 in metabolic dysfunction-associated steatotic liver disease[J]. Med J Peking Union Med Coll Hosp, 2024, 15( 5): 1117- 1123. DOI: 10.12290/xhyxzz.2024-0035.董凯旋, 陈丹妮, 郑亚, 等. Ceacam1在代谢功能障碍相关脂肪性肝病中的作用[J]. 协和医学杂志, 2024, 15( 5): 1117- 1123. DOI: 10.12290/xhyxzz.2024-0035. [13] BERGMAN RN, KABIR M, ADER M. The physiology of insulin clearance[J]. Int J Mol Sci, 2022, 23( 3): 1826. DOI: 10.3390/ijms23031826. [14] BRIL F, BARB D, PORTILLO-SANCHEZ P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease[J]. Hepatology, 2017, 65( 4): 1132- 1144. DOI: 10.1002/hep.28985. [15] BRIL F, LOMONACO R, ORSAK B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis[J]. Hepatology, 2014, 59( 6): 2178- 2187. DOI: 10.1002/hep.26988. [16] ELKRIEF L, RAUTOU PE, SARIN S, et al. Diabetes mellitus in patients with cirrhosis: Clinical implications and management[J]. Liver Int, 2016, 36( 7): 936- 948. DOI: 10.1111/liv.13115. [17] NAGAISHI T, PAO L, LIN SH, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms[J]. Immunity, 2006, 25( 5): 769- 781. DOI: 10.1016/j.immuni.2006.08.026. [18] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [19] NIAN FL, LU XL. Non-alcoholic fatty liver disease and diabetes mellitus type 2[J]. J Prac Hepatol, 2022, 25( 3): 314- 317. DOI: 10.3969/j.issn.1672-5069.2022.03.003.年福临, 鲁晓岚. 糖尿病与非酒精性脂肪性肝病[J]. 实用肝脏病杂志, 2022, 25( 3): 314- 317. DOI: 10.3969/j.issn.1672-5069.2022.03.003. [20] SHEN ZZ, ZHANG HY, LIU LX. Research progress of metabolic dysfunction related fatty liver disease[J]. Anhui Med Pharm J, 2024, 28( 8): 1496- 1502. DOI: 10.3969/j.issn.1009-6469.2024.08.003.沈震洲, 张海燕, 刘立新. 代谢功能障碍相关脂肪肝疾病的研究进展[J]. 安徽医药, 2024, 28( 8): 1496- 1502. DOI: 10.3969/j.issn.1009-6469.2024.08.003. [21] ESCOBAR O, MIZUMA H, SOTHERN MS, et al. Hepatic insulin clearance increases after weight loss in obese children and adolescents[J]. Am J Med Sci, 1999, 317( 5): 282- 286. DOI: 10.1097/00000441-199905000-00003. [22] GIUGLIANO D, QUATRARO A, MINEI A, et al. Hyperinsulinemia in hypertension: Increased secretion, reduced clearance or both?[J]. J Endocrinol Invest, 1993, 16( 5): 315- 321. DOI: 10.1007/BF03348843. [23] HANSEN BC, STRIFFLER JS, BODKIN NL. Decreased hepatic insulin extraction precedes overt noninsulin dependent(Type II)diabetes in obese monkeys[J]. Obes Res, 1993, 1( 4): 252- 260. DOI: 10.1002/j.1550-8528.1993.tb00619.x. [24] POY MN, YANG Y, REZAEI K, et al. CEACAM1 regulates insulin clearance in liver[J]. Nat Genet, 2002, 30( 3): 270- 276. DOI: 10.1038/ng840. [25] HELAL RA, RUSSO L, GHADIEH HE, et al. Regulation of hepatic fibrosis by carcinoembryonic antigen-related cell adhesion molecule 1[J]. Metabolism, 2021, 121: 154801. DOI: 10.1016/j.metabol.2021.154801. [26] KIM SP, ELLMERER M, KIRKMAN EL, et al. Beta-cell“rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model[J]. Am J Physiol Endocrinol Metab, 2007, 292( 6): E1581- E1589. DOI: 10.1152/ajpendo.00351.2006. [27] GASTALDELLI A, ABDUL GHANI M, DEFRONZO RA. Adaptation of insulin clearance to metabolic demand is a key determinant of glucose tolerance[J]. Diabetes, 2021, 70( 2): 377- 385. DOI: 10.2337/db19-1152. [28] DEBOSE-BOYD RA, YE J. SREBPs in lipid metabolism, insulin signaling, and beyond[J]. Trends Biochem Sci, 2018, 43( 5): 358- 368. DOI: 10.1016/j.tibs.2018.01.005. [29] NAJJAR SM, YANG Y, FERNSTRÖM MA, et al. Insulin acutely decreases hepatic fatty acid synthase activity[J]. Cell Metab, 2005, 2( 1): 43- 53. DOI: 10.1016/j.cmet.2005.06.001. [30] RAMAKRISHNAN SK, KHUDER SS, AL-SHARE QY, et al. PPARα(peroxisome proliferator-activated receptor α)activation reduces hepatic CEACAM1 protein expression to regulate fatty acid oxidation during fasting-refeeding transition[J]. J Biol Chem, 2016, 291( 15): 8121- 8129. DOI: 10.1074/jbc.M116.714014. [31] RAMAKRISHNAN SK, RUSSO L, GHANEM SS, et al. Fenofibrate decreases insulin clearance and insulin secretion to maintain insulin sensitivity[J]. J Biol Chem, 2016, 291( 46): 23915- 23924. DOI: 10.1074/jbc.M116.745778. [32] MATSUBAYASHI Y, YOSHIDA A, SUGANAMI H, et al. Role of fatty liver in the association between obesity and reduced hepatic insulin clearance[J]. Diabetes Metab, 2018, 44( 2): 135- 142. DOI: 10.1016/j.diabet.2017.12.003. [33] SVEDBERG J, BJÖRNTORP P, SMITH U, et al. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes[J]. Diabetes, 1990, 39( 5): 570- 574. DOI: 10.2337/diab.39.5.570. [34] MITTELMAN SD, van CITTERS GW, KIM SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response[J]. Diabetes, 2000, 49( 12): 2116- 2125. DOI: 10.2337/diabetes.49.12.2116. [35] SVEDBERG J, STRÖMBLAD G, WIRTH A, et al. Fatty acids in the portal vein of the rat regulate hepatic insulin clearance[J]. J Clin Invest, 1991, 88( 6): 2054- 2058. DOI: 10.1172/JCI115534. [36] TAN M, ZHANG H, REN JH, et al. The role of lipid metabolism disorders in non-alcoholic fatty liver disease[J]. Chin J Gastroenterol Hepatol, 2024, 33( 8): 1082- 1086. DOI: 10. 3969/ j. issn. 1006-5709. 2024. 08. 025.谭明, 张慧, 任吉华, 等. 脂质代谢紊乱在非酒精性脂肪性肝病中的作用概述[J]. 胃肠病和肝病学杂志, 2024, 33( 8): 1082- 1086. DOI: 10. 3969/j. issn. 1006-5709. 2024. 08. 025. [37] ZHOU LL, SHEN HY, LI XF, et al. Endoplasmic reticulum stress in innate immune cells-a significant contribution to non-alcoholic fatty liver disease[J]. Front Immunol, 2022, 13: 951406. DOI: 10.3389/fimmu.2022.951406. [38] PARK DJ, SUNG PS, KIM JH, et al. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1[J]. J Immunother Cancer, 2020, 8( 1): e000301. DOI: 10.1136/jitc-2019-000301. [39] KHAIRNAR V, DUHAN V, PATIL AM, et al. CEACAM1 promotes CD8+ T cell responses and improves control of a chronic viral infection[J]. Nat Commun, 2018, 9: 2561. DOI: 10.1038/s41467-018-04832-2. [40] IIJIMA H, NEURATH MF, NAGAISHI T, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1[J]. J Exp Med, 2004, 199( 4): 471- 482. DOI: 10.1084/jem.20030437. [41] HORST AK, NAJJAR SM, WAGENER C, et al. CEACAM1 in liver injury, metabolic and immune regulation[J]. Int J Mol Sci, 2018, 19( 10): 3110. DOI: 10.3390/ijms19103110. [42] ULLAH R, RAUF N, NABI G, et al. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: Recent updates[J]. Int J Biol Sci, 2019, 15( 2): 265- 276. DOI: 10.7150/ijbs.30121. -



 PDF下载 ( 842 KB)
PDF下载 ( 842 KB)

 下载:
下载:


