基于腺苷酸活化蛋白激酶/Unc-51样自噬激活激酶1信号通路探讨高原低氧适应对肝缺血再灌注损伤大鼠模型的保护作用
DOI: 10.12449/JCH250725
Protective effect of high-altitude hypoxia acclimatization against hepatic ischemia-reperfusion injury in rats: A study based on the adenosine monophosphate-activated protein kinase/Unc-51 like autophagy activating kinase 1 signaling pathway
-
摘要:
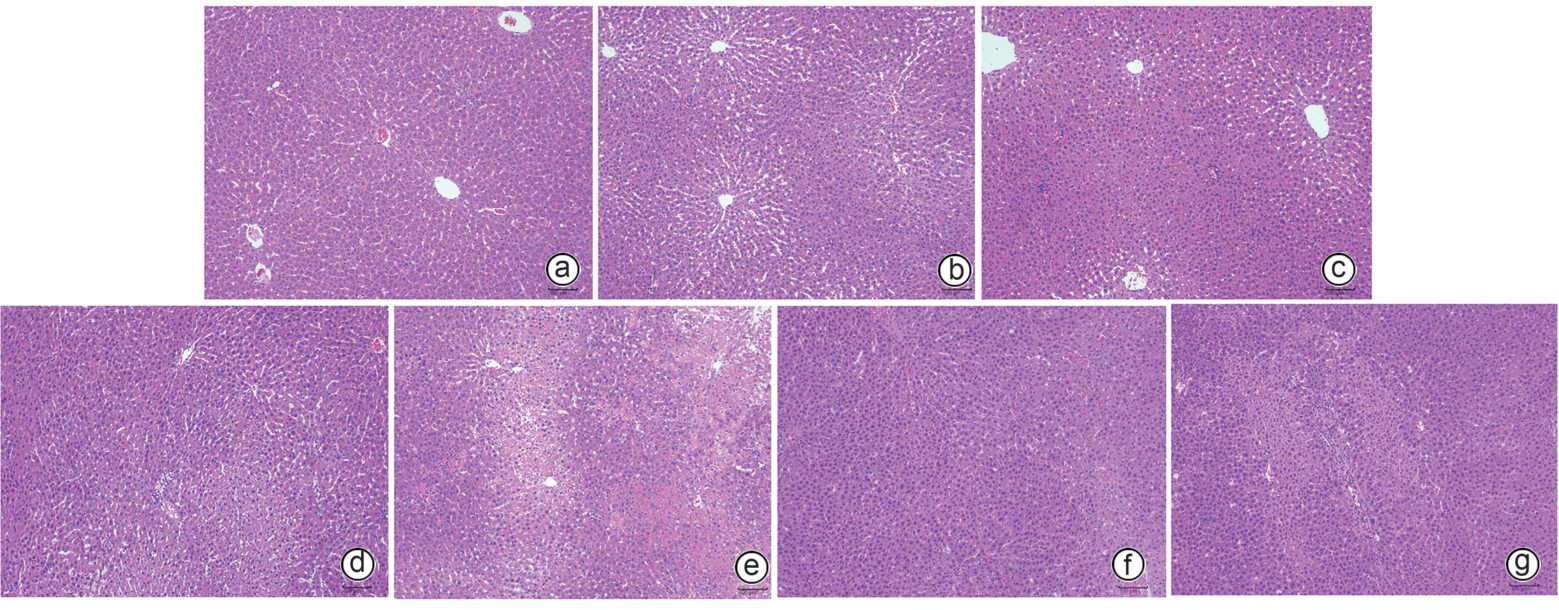
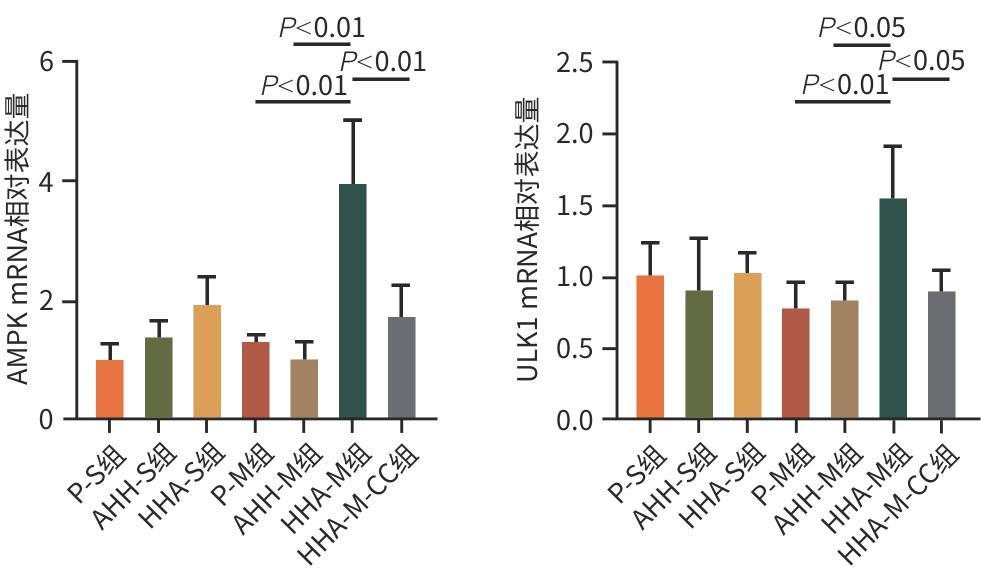
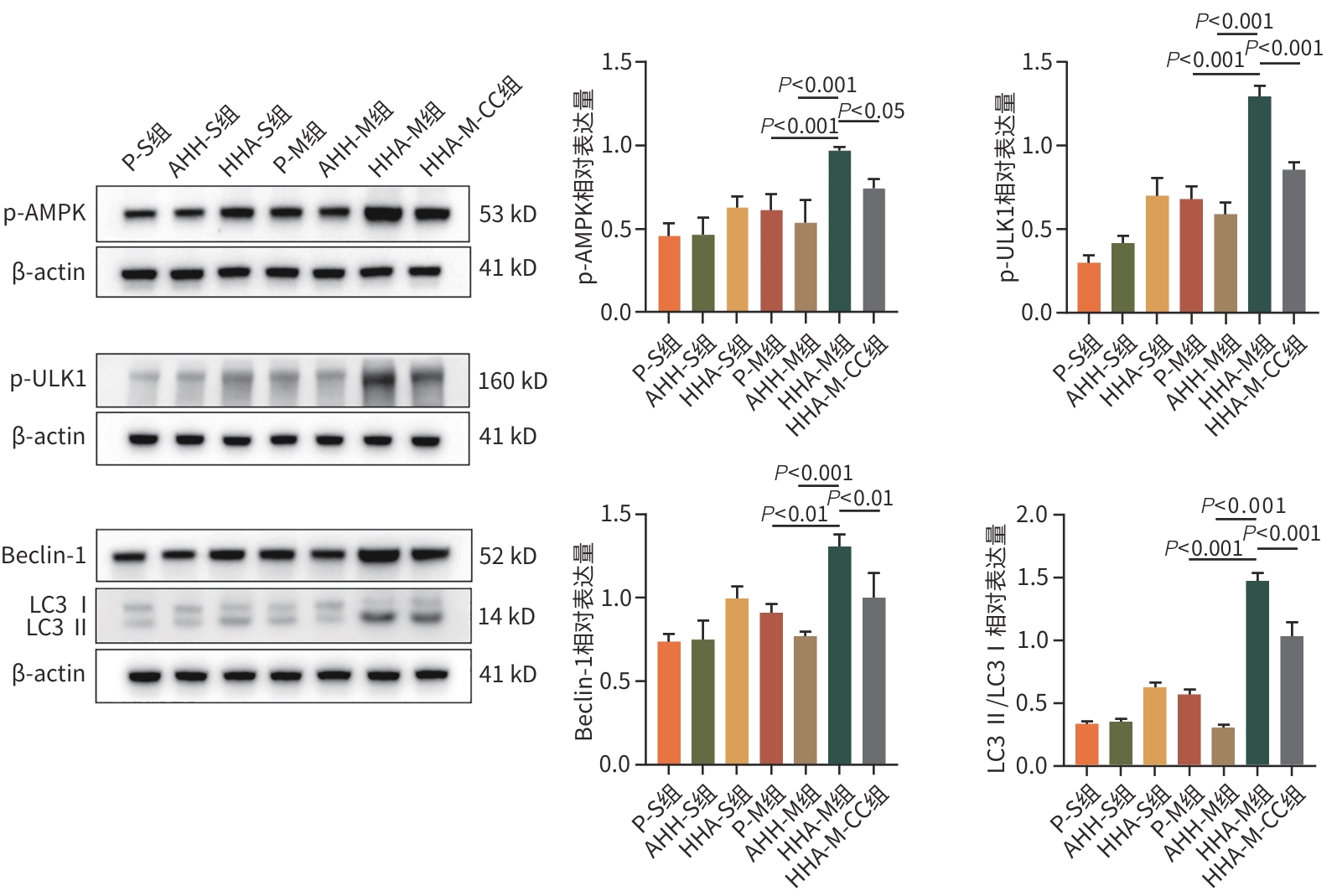
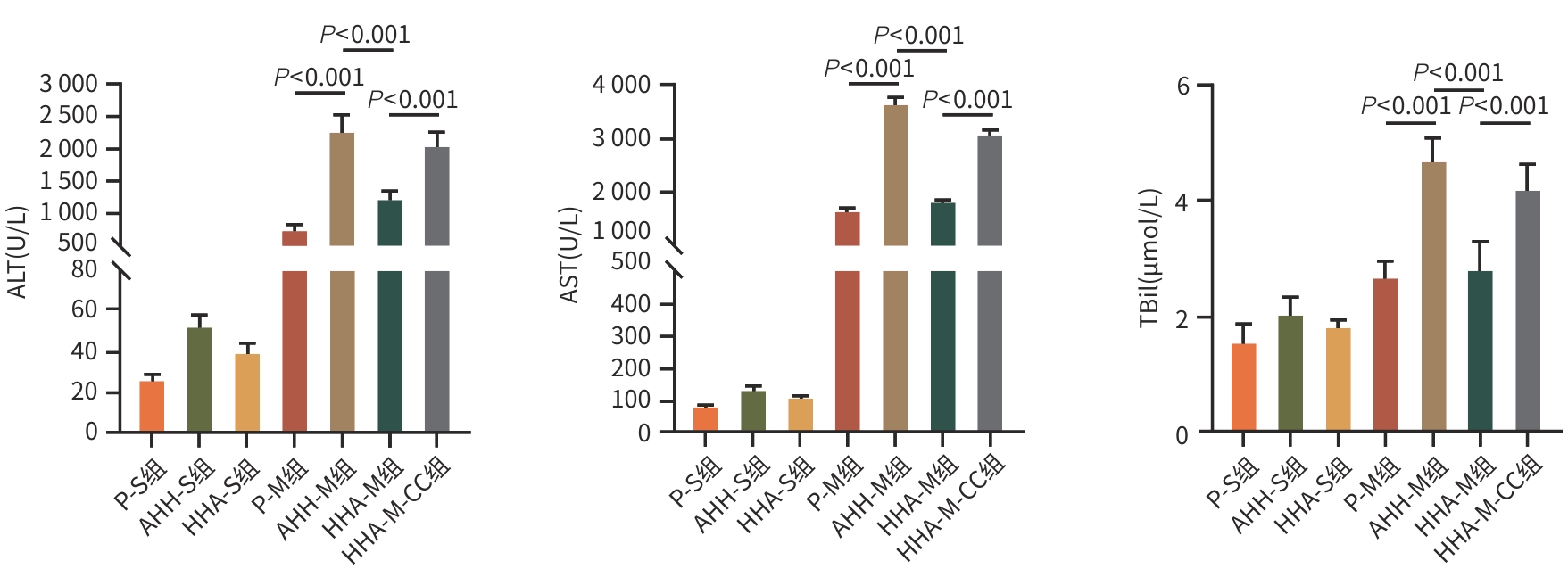
目的 观察高原低氧适应对肝缺血再灌注损伤(HIRI)大鼠模型的保护作用,探索高原低氧适应激活自噬的作用机制。 方法 将56只SD雄性大鼠随机分为以下7组:平原假手术组(P-S)、平原模型组(P-M)、急性高原低氧假手术组(AHH-S)、急性高原低氧模型组(AHH-M)、高原低氧适应假手术组(HHA-S)、高原低氧适应模型组(HHA-M),以及含AMPK抑制剂复合物C(CC)的高原低氧适应模型组(HHA-M-CC),每组8只。急性高原低氧组和高原低氧适应组分别将大鼠置于海拔5 000米的低压氧舱中1周和12周;假手术组仅开腹暴露肝门静脉,未做血管夹闭处理;HHA-M-CC组术前1 h行腹腔注射20 mg/kg剂量CC,其余组注射等体积生理盐水。采用全自动生化分析仪检测肝功能指标ALT、AST、TBil水平;苏木素-伊红染色观察肝组织病理改变;透射电镜观察肝组织自噬体形成情况;RT-qPCR检测肝脏腺苷酸活化蛋白激酶(AMPK)、Unc-51样自噬激活激酶1(ULK1)mRNA表达水平;Western Blot技术检测磷酸化腺苷酸蛋白激酶(p-AMPK)、磷酸化UNC-51样激酶(p-ULK1)、Beclin-1、微管相关蛋白1轻链3Ⅱ型(LC3Ⅱ)蛋白的表达水平。计量资料多组间比较采用方差分析,进一步两两比较采用LSD-t检验。 结果 与AHH-M组和HHA-M-CC组比较,HHA-M组ALT、AST、TBil水平明显降低(P值均<0.05),肝组织病理损伤减弱,Suzuki评分显著降低(P值均<0.05),透射电镜下肝细胞形态结构异常程度减弱,自噬体数量明显增多,AMPK、ULK1 mRNA表达水平均明显上升(P值均<0.05),p-AMPK、p-ULK1、Beclin-1、LC3Ⅱ蛋白表达明显上调(P值均<0.05)。 结论 高原低氧适应可通过AMPK/ULK1信号通路相关蛋白的激活,增强肝细胞的自噬作用,进而缓解SD大鼠HIRI。 -
关键词:
- 低氧 /
- 再灌注损伤 /
- 自噬 /
- 大鼠, Sprague-Dawley
Abstract:Objective To investigate the protective effect of high-altitude hypoxia acclimatization against hepatic ischemia-reperfusion injury (HIRI) in rats, as well as the mechanism of action of high-altitude hypoxia acclimatization in activating autophagy. Methods A total of 56 male Sprague-Dawley rats were randomly divided into plain sham-operation group (P-S group), plain model group (P-M group), acute high-altitude hypoxia sham-operation group (AHH-S group), acute high-altitude hypoxia model group (AHH-M group), high-altitude hypoxia acclimatization sham-operation group (HHA-S group), high-altitude hypoxia acclimatization model group (HHA-M group), and high-altitude hypoxia acclimatization model group with the adenosine monophosphate-activated protein kinase (AMPK) inhibitor compound C (HHA-M-CC group), with 8 rats in each group. The rats in the acute high-altitude hypoxia groups and the high-altitude hypoxia acclimatization groups were placed in a low-pressure oxygen chamber at an altitude of 5 000 meters for 1 week and 12 weeks, respectively; the rats in the sham-operation groups were given laparotomy to expose the portal vein without vascular clamping; the rats in the HHA-M-CC group were given abdominal injection of 20 mg/kg CC at 1 hour before surgery, while those in the other groups were given injection of an equal volume of normal saline. An automatic biochemical analyzer was used to measure the levels of liver function parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil); HE staining was used to observe liver histopathological changes; transmission electron microscopy was used to observe the formation of autophagosomes in liver tissue; RT-qPCR was used to measure the mRNA expression levels of AMPK and Unc-51 like autophagy activating kinase 1 (ULK1) in liver tissue; Western Blot was used to measure the protein expression levels of phosphorylated AMPK (p-AMPK), phosphorylated ULK1 (p-ULK1), Beclin-1, and microtubule-associated protein 1 light chain 3 Ⅱ (LC3Ⅱ). An analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was sued for comparison between two groups. Results Compared with the AHH-M and HHA-M-CC groups, the HHA-M group had significantly reductions in the levels of ALT, AST, and TBil (all P<0.05), alleviation of liver histopathological injury, a significant reduction in Suzuki score (all P<0.05), a reduction in the degree of abnormal morphological structure of hepatocytes under transmission electron microscopy, and significant increases in the number of autophagosomes, the mRNA expression levels of AMPK and ULK1 (all P<0.05), and the protein expression levels of p-AMPK, p-ULK1, Beclin-1, and LC3Ⅱ (all P<0.05). Conclusion High-altitude hypoxia acclimatization can alleviate HIRI in SD rats by activating the AMPK/ULK1 signaling pathway and enhancing autophagy in hepatocytes. -
Key words:
- Hypoxia /
- Reperfusion Injury /
- Autophagy /
- Rats, Sprague-Dawley
-
表 1 引物序列
Table 1. Primer sequence
基因 上游(5'-3') 下游(5'-3') β-actin GGGAAATCGTGCGTGA‑
CATTGCGGCAGTGGCCATC‑
TCAMPK ATGATGAGGTGGTGGA‑
GCAGAGGGGTTCTCGGCTGTGCT‑
GGAATCULK1 TACACAGCAAGGGCAT‑
CATTCACCCGGGCAAATCCAAAG‑
TCAGCAATC -
[1] KHURANA P, GUPTA A, SUGADEV R, et al. HAHmiR.DB: A server platform for high-altitude human miRNA-gene coregulatory networks and associated regulatory circuits[J]. Database(Oxford), 2020, 2020: baaa101. DOI: 10.1093/database/baaa101. [2] BIAN SZ, ZHANG JH, GAO XB, et al. Risk factors for high-altitude headache upon acute high-altitude exposure at 3 700 m in young Chinese men: A cohort study[J]. J Headache Pain, 2013, 14( 1): 35. DOI: 10.1186/1129-2377-14-35. [3] DONKOR N, GARDNER JJ, BRADSHAW JL, et al. Ocular inflammation and oxidative stress as a result of chronic intermittent hypoxia: A rat model of sleep apnea[J]. Antioxidants(Basel), 2024, 13( 7): 878. DOI: 10.3390/antiox13070878. [4] MIALET-PEREZ J, BELAIDI E. Interplay between hypoxia inducible Factor-1 and mitochondria in cardiac diseases[J]. Free Radic Biol Med, 2024, 221: 13- 22. DOI: 10.1016/j.freeradbiomed.2024.04.239. [5] ZHOU Y, HE LN, WANG LN, et al. Human amniotic mesenchymal stromal cell-derived exosomes promote neuronal function by inhibiting excessive apoptosis in a hypoxia/ischemia-induced cerebral palsy model: A preclinical study[J]. Biomed Pharmacother, 2024, 173: 116321. DOI: 10.1016/j.biopha.2024.116321. [6] MARTINEZ-CANTON M, GALVAN-ALVAREZ V, GALLEGO-SELLES A, et al. Activation of macroautophagy and chaperone-mediated autophagy in human skeletal muscle by high-intensity exercise in normoxia and hypoxia and after recovery with or without post-exercise ischemia[J]. Free Radic Biol Med, 2024, 222: 607- 624. DOI: 10.1016/j.freeradbiomed.2024.07.012. [7] ALSUP C, LIPMAN GS, POMERANZ D, et al. Interstitial pulmonary edema assessed by lung ultrasound on ascent to high altitude and slight association with acute mountain sickness: A prospective observational study[J]. High Alt Med Biol, 2019, 20( 2): 150- 156. DOI: 10.1089/ham.2018.0123. [8] LI WH, LI YX, REN J. High altitude hypoxia on brain ultrastructure of rats and Hsp70 expression changes[J]. Br J Neurosurg, 2019, 33( 2): 192- 195. DOI: 10.1080/02688697.2018.1519108. [9] FENG ZL, ZHAO T, CHENG X, et al. Effects of simulated high-altitude hypobaric hypoxia on cardiac structure and function in rats[J]. Chin J Appl Physiol, 2019, 35( 2): 173- 177, 4. DOI: 10.12047/j.cjap.5751.2019.038.冯振龙, 赵彤, 成祥, 等. 模拟高原低压低氧环境对大鼠心脏结构和功能影响[J]. 中国应用生理学杂志, 2019, 35( 2): 173- 177, 4. DOI: 10.12047/j.cjap.5751.2019.038. [10] QU XY, YANG T, WANG X, et al. Macrophage RIPK3 triggers inflammation and cell death via the XBP1-Foxo1 axis in liver ischaemia-reperfusion injury[J]. JHEP Rep, 2023, 5( 11): 100879. DOI: 10.1016/j.jhepr.2023.100879. [11] WANG JH, AHN IS, FISCHER TD, et al. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice[J]. Gastroenterology, 2011, 141( 6): 2188- 2199. e 6. DOI: 10.1053/j.gastro.2011.08.005. [12] LIU JJ, HAO HJ, HUANG H, et al. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy[J]. Int J Low Extrem Wounds, 2015, 14( 1): 63- 72. DOI: 10.1177/1534734615573660. [13] FAN WS, HAN D, SUN ZC, et al. Endothelial deletion of mTORC1 protects against hindlimb ischemia in diabetic mice via activation of autophagy, attenuation of oxidative stress and alleviation of inflammation[J]. Free Radic Biol Med, 2017, 108: 725- 740. DOI: 10.1016/j.freeradbiomed.2017.05.001. [14] DELBRIDGE LMD, MELLOR KM, TAYLOR DJ, et al. Myocardial stress and autophagy: Mechanisms and potential therapies[J]. Nat Rev Cardiol, 2017, 14( 7): 412- 425. DOI: 10.1038/nrcardio.2017.35. [15] DAI SH, CHEN T, LI X, et al. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway[J]. Free Radic Biol Med, 2017, 108: 345- 353. DOI: 10.1016/j.freeradbiomed.2017.04.005. [16] PU T, LIAO XH, SUN H, et al. Augmenter of liver regeneration regulates autophagy in renal ischemia-reperfusion injury via the AMPK/mTOR pathway[J]. Apoptosis, 2017, 22( 7): 955- 969. DOI: 10.1007/s10495-017-1370-6. [17] MOHAMED DZ, EL-SISI AEE, SOKAR SS, et al. Targeting autophagy to modulate hepatic ischemia/reperfusion injury: A comparative study between octreotide and melatonin as autophagy modulators through AMPK/PI3K/AKT/mTOR/ULK1 and Keap1/Nrf2 signaling pathways in rats[J]. Eur J Pharmacol, 2021, 897: 173920. DOI: 10.1016/j.ejphar.2021.173920. [18] LIGGETT JR, KANG JM, RANJIT S, et al. Oral N-acetylcysteine decreases IFN-γ production and ameliorates ischemia-reperfusion injury in steatotic livers[J]. Front Immunol, 2022, 13: 898799. DOI: 10.3389/fimmu.2022.898799. [19] OLTHOF PB, van GOLEN RF, MEIJER B, et al. Warm ischemia time-dependent variation in liver damage, inflammation, and function in hepatic ischemia/reperfusion injury[J]. Biochim Biophys Acta Mol Basis Dis, 2017, 1863( 2): 375- 385. DOI: 10.1016/j.bbadis.2016.10.022. [20] CARDINAL J, PAN PH, TSUNG A. Protective role of cisplatin in ischemic liver injury through induction of autophagy[J]. Autophagy, 2009, 5( 8): 1211- 1212. DOI: 10.4161/auto.5.8.9972. [21] RAUTOU PE, MANSOURI A, LEBREC D, et al. Autophagy in liver diseases[J]. J Hepatol, 2010, 53( 6): 1123- 1134. DOI: 10.1016/j.jhep.2010.07.006. [22] RICKENBACHER A, JANG JH, LIMANI P, et al. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice[J]. J Hepatol, 2014, 61( 2): 301- 308. DOI: 10.1016/j.jhep.2014.04.010. [23] WANG DW, MA Y, LI ZT, et al. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice[J]. Autophagy, 2012, 8( 6): 954- 962. DOI: 10.4161/auto.19927. [24] HU J, ZHU XH, ZHANG XJ, et al. Targeting TRAF3 signaling protects against hepatic ischemia/reperfusions injury[J]. J Hepatol, 2016, 64( 1): 146- 159. DOI: 10.1016/j.jhep.2015.08.021. [25] SCHOENE RB. Illnesses at high altitude[J]. Chest, 2008, 134( 2): 402- 416. DOI: 10.1378/chest.07-0561. [26] IRARRÁZAVAL S, ALLARD C, CAMPODÓNICO J, et al. Oxidative stress in acute hypobaric hypoxia[J]. High Alt Med Biol, 2017, 18( 2): 128- 134. DOI: 10.1089/ham.2016.0119. [27] YUHAI GU, ZHEN Z. Significance of the changes occurring in the levels of interleukins, SOD and MDA in rat pulmonary tissue following exposure to different altitudes and exposure times[J]. Exp Ther Med, 2015, 10( 3): 915- 920. DOI: 10.3892/etm.2015.2604. [28] HU YJ, SUN Q, LI ZP, et al. High basal level of autophagy in high-altitude residents attenuates myocardial ischemia-reperfusion injury[J]. J Thorac Cardiovasc Surg, 2014, 148( 4): 1674- 1680. DOI: 10.1016/j.jtcvs.2014.03.038. [29] KANG JW, CHO HI, LEE SM. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion[J]. Cell Physiol Biochem, 2014, 33( 1): 23- 36. DOI: 10.1159/000356647. [30] CZAJA MJ. Functions of autophagy in hepatic and pancreatic physiology and disease[J]. Gastroenterology, 2011, 140( 7): 1895- 1908. DOI: 10.1053/j.gastro.2011.04.038. [31] XIN DQ, CHU XL, BAI XM, et al. L-Cysteine suppresses hypoxia-ischemia injury in neonatal mice by reducing glial activation, promoting autophagic flux and mediating synaptic modification via H2S formation[J]. Brain Behav Immun, 2018, 73: 222- 234. DOI: 10.1016/j.bbi.2018.05.007. [32] SONG DD, ZHANG TT, CHEN JL, et al. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain[J]. Cell Death Dis, 2017, 8( 7): e2912. DOI: 10.1038/cddis.2017.289. [33] XIE Y, JIANG DF, XIAO J, et al. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway[J]. Cell Death Dis, 2018, 9( 3): 338. DOI: 10.1038/s41419-018-0358-7. [34] ZHENG Z, ZHANG L, QU Y, et al. Mesenchymal stem cells protect against hypoxia-ischemia brain damage by enhancing autophagy through brain derived neurotrophic factor/mammalin target of rapamycin signaling pathway[J]. Stem Cells, 2018, 36( 7): 1109- 1121. DOI: 10.1002/stem.2808. [35] HU YY, ZHANG X, LUO Y, et al. Advances in the protective mechanism and clinical implications of autophagy in liver failure[J]. J Clin Hepatol, 2023, 39( 10): 2485- 2490. DOI: 10.3969/j.issn.1001-5256.2023.10.030.胡洋洋, 张兴, 罗越, 等. 自噬对肝衰竭的保护作用机制与临床价值[J]. 临床肝胆病杂志, 2023, 39( 10): 2485- 2490. DOI: 10.3969/j.issn.1001-5256.2023.10.030. [36] PAN M, SHI XY. Role of mitophagy in the development and progression of liver-related diseases[J]. J Clin Hepatol, 2024, 40( 2): 413- 418. DOI: 10.12449/JCH240232.潘萌, 史晓燕. 线粒体自噬在肝脏相关疾病发生发展中的作用[J]. 临床肝胆病杂志, 2024, 40( 2): 413- 418. DOI: 10.12449/JCH240232. [37] PEI CX, SHEN ZR, WU YC, et al. Eleutheroside B pretreatment attenuates hypobaric hypoxia-induced high-altitude pulmonary edema by regulating autophagic flux via the AMPK/mTOR pathway[J]. Phytother Res, 2024, 38( 12): 5657- 5671. DOI: 10.1002/ptr.8333. [38] STEINBERG GR, HARDIE DG. New insights into activation and function of the AMPK[J]. Nat Rev Mol Cell Biol, 2023, 24: 255- 272. DOI: 10.1038/s41580-022-00547-x. [39] GUO F, WEN WL, MI ZP, et al. NRSN2 promotes the malignant behavior of HPV-transfected laryngeal carcinoma cells through AMPK/ULK1 pathway mediated autophagy activation[J]. Cancer Biol Ther, 2024, 25( 1): 2334463. DOI: 10.1080/15384047.2024.2334463. -



 PDF下载 ( 3041 KB)
PDF下载 ( 3041 KB)

 下载:
下载: