牙髓间充质干细胞治疗自身免疫性肝炎小鼠模型的效果及其免疫调控机制
DOI: 10.12449/JCH250719
伦理学声明:本研究方案于2023年6月14日经由首都医科大学附属北京积水潭医院动物研究委员会批准,批号:202306006,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:李茵负责设计论文框架,起草论文;宋光元负责实验操作,研究过程的实施; 李晓东负责数据收集,统计学分析、绘制图表;史婉婉负责论文修改;王贵强负责拟定写作思路,指导撰写文章并最后定稿。
Therapeutic effects of dental pulp stem cells in a mouse model of autoimmune hepatitis and related immunoregulatory mechanisms
-
摘要:
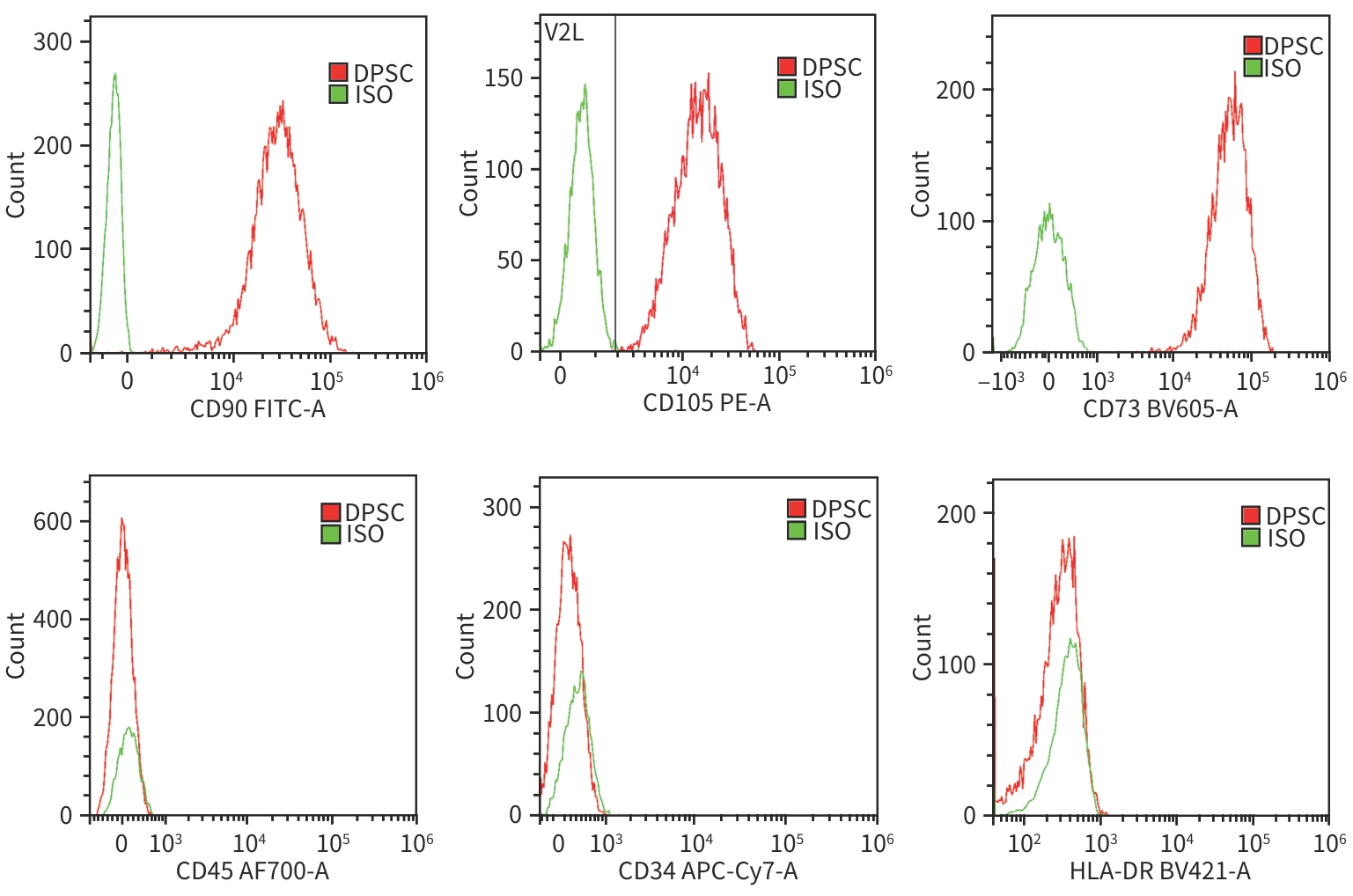
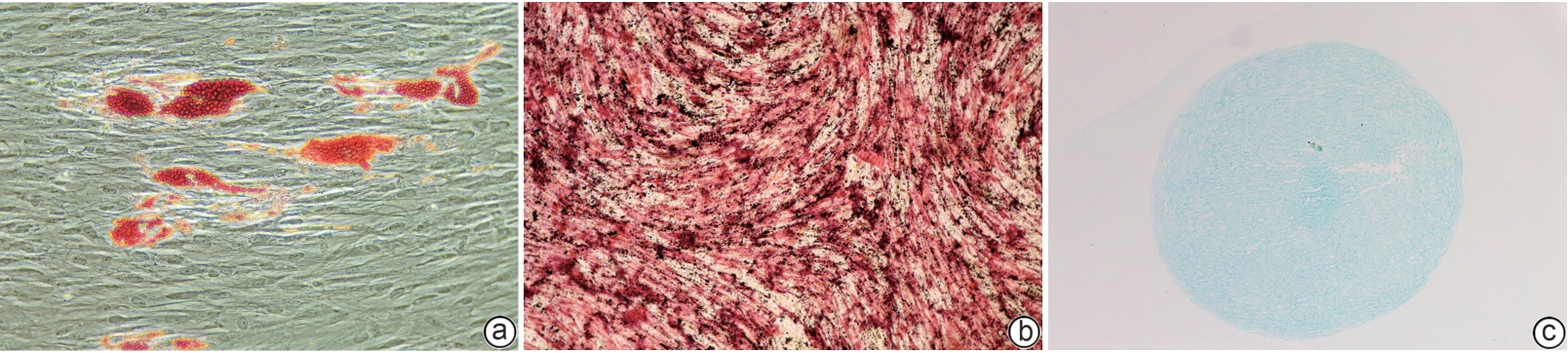
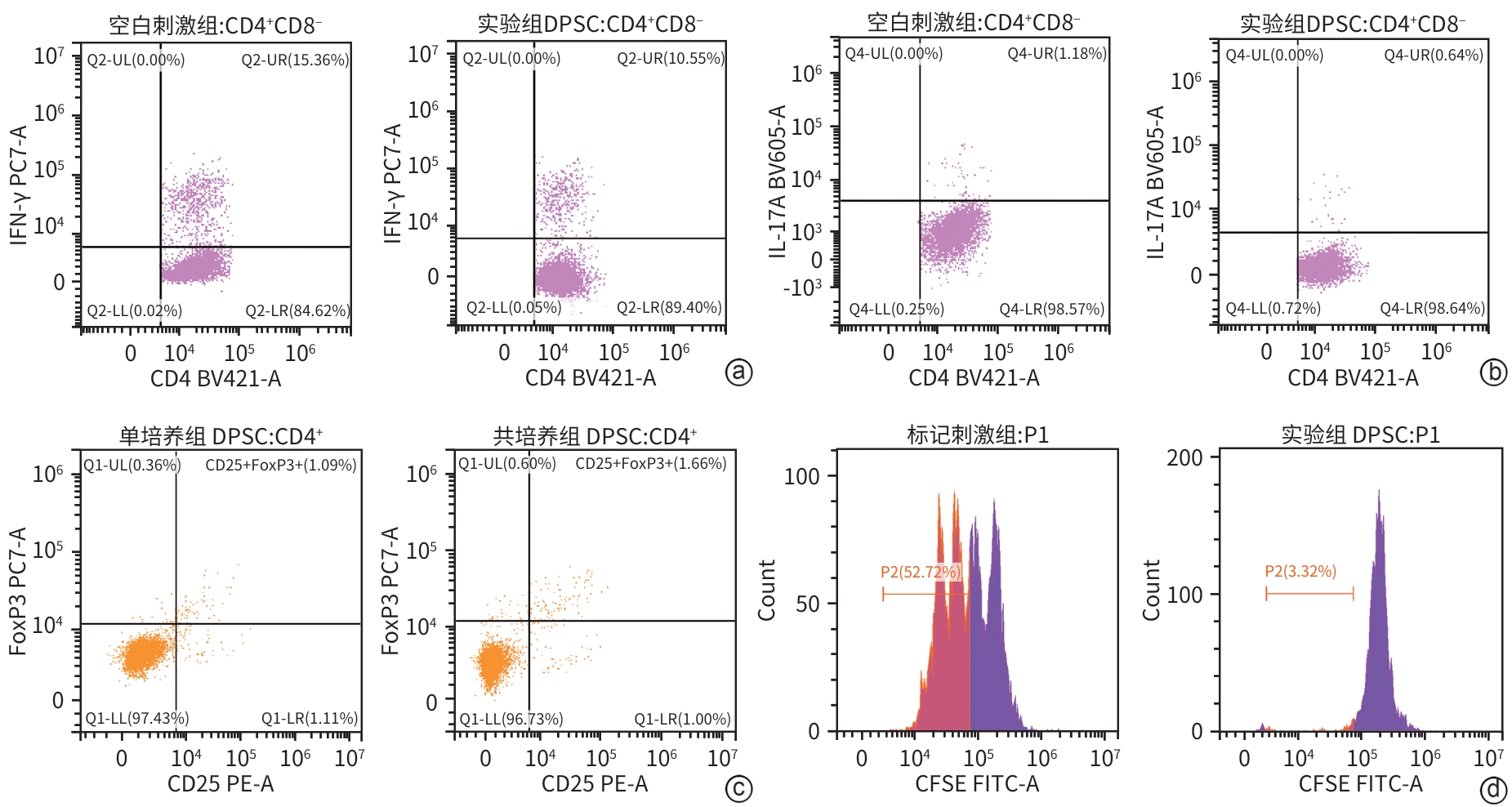
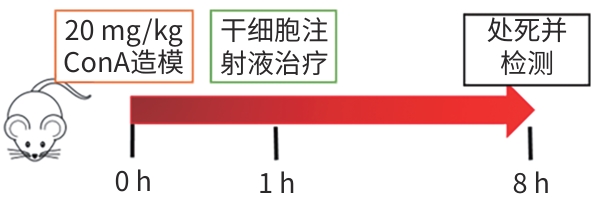
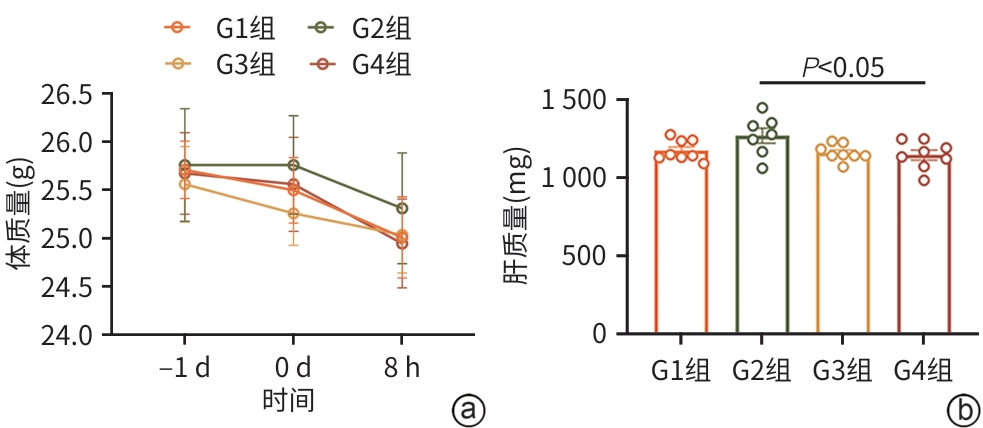
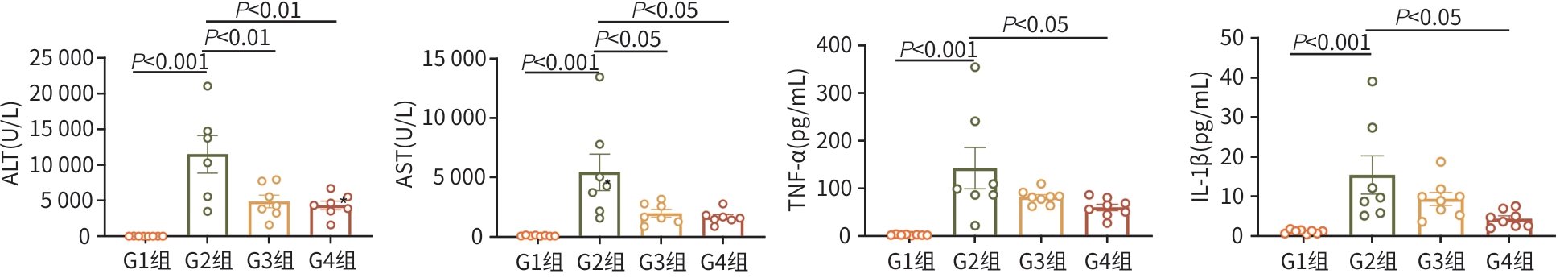
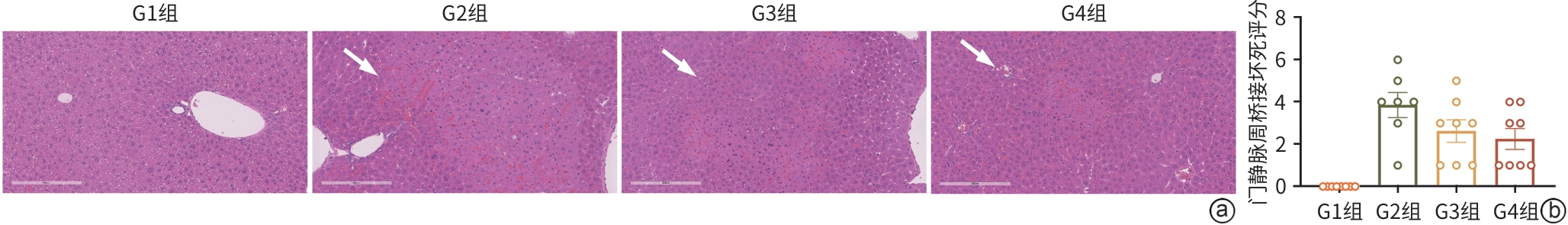
目的 探讨牙髓间充质干细胞(DPSC)在体内外实验中对自身免疫性肝炎的治疗作用及相关机制。 方法 首先通过体外共培养体系评估DPSC的免疫调节作用;然后进行动物实验,将32只小鼠随机分成健康对照组、模型组、阳性药物组、DPSC治疗组,每组8只。检测各组血清ALT、AST及炎症因子水平,HE染色评估肝脏病理损伤。计量资料符合正态分布多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 体外实验结果显示DPSC的CD105、CD73和CD90阳性率分别为99.97%、100%和99.53%;而CD34、HLA-DR和CD45阳性率分别为0.56%、0.17%和0,符合间充质干细胞特征。DPSC可显著抑制辅助性T细胞(Th)1和Th17的增殖,抑制率分别为31.32%、45.76%;DPSC可促进调节性T细胞(Treg)(CD4+CD25+FoxP3+)的增殖,促进率为52.29%。DPSC对淋巴细胞的增殖抑制率为93.70%。在自身免疫性肝炎小鼠模型中,DPSC治疗组小鼠血清ALT、AST水平较模型组显著下降66.8%和60.0%(t值分别为3.321、2.907,P值分别为0.007 5、0.017 5),炎症相关因子TNF-α和IL-1β显著下降57.5%和71.3%(t值分别为2.484、2.796,P值分别为0.039 8、0.020 6),组织病理学结果显示门静脉周桥接坏死改善不明显(t=1.969,P=0.098)。 结论 DPSC通过免疫调节有效缓解免疫性肝损伤,为临床转化提供了实验依据。 -
关键词:
- 间质干细胞 /
- 牙髓 /
- 肝炎, 自身免疫性 /
- 小鼠, 近交BALB/C /
- 免疫调节
Abstract:Objective To investigate the therapeutic effect of dental pulp stem cells (DPSCs) on autoimmune hepatitis in invivo and in vitro experiments and the related mechanism. Methods An in vitro co-culture system was used to evaluate the immunoregulatory effect of DPSCs, and 32 mice were randomly divided into healthy control group, model group, positive drug group, and DPSCs treatment group, with 8 mice in each group. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and inflammatory factors were measured, and HE staining was used to assess liver pathological injury. An analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results The in vitro experiment showed that the positive rates of CD105, CD73, and CD90 in DPSCs were 99.97%, 100%, and 99.53%, respectively, while the positive rates of CD34, HLA-DR, and CD45 were 0.56%, 0.17%, and 0, respectively. DPSCs significantly inhibited the proliferation of Th1 and Th17 subsets, with inhibition rates of 31.32% and 45.76%, respectively; DPSCs promoted the proliferation of Treg (CD4+CD25+FoxP3+), with a promoting rate of 52.29%. DPSCs had an inhibition rate of 93.70% on the proliferation of lymphocytes. In the mouse model of autoimmune hepatitis, compared with the model group, the DPSCs treatment group had significant reductions in the serum levels of ALT and AST, with reduction rates of 66.8% and 60.0%, respectively (t=3.321 and 2.907, P=0.007 5 and 0.017 5) and significant reductions in the inflammatory factors tumor necrosis factor-α and interleukin-1β, with reduction rates of 57.5% and 71.3%, respectively (t=2.484 and 2.796, P=0.039 8 and 0.020 6), and histopathological examination showed no significant improvement in periportal bridging necrosis (t=1.969, P=0.098). Conclusion DPSCs effectively alleviate immune-mediated liver injury through immunoregulation, which provides an experimental basis for clinical translation. -
Key words:
- Mesenchymal Stem Cells /
- Dental Pulp /
- Hepatitis, Autoimmune /
- Mice, Inbred BALB C /
- Immunoregulation
-
-
[1] KERKAR N, CHAN A. Autoimmune hepatitis, sclerosing cholangitis, and autoimmune sclerosing cholangitis or overlap syndrome[J]. Clin Liver Dis, 2018, 22( 4): 689- 702. DOI: 10.1016/j.cld.2018.06.005. [2] VOLK ML, REAU N. Diagnosis and management of autoimmune hepatitis in adults and children: A patient-friendly summary of the 2019 AASLD guidelines[J]. Clin Liver Dis(Hoboken), 2021, 17( 2): 85- 89. DOI: 10.1002/cld.1080. [3] LOHSE AW, SEBODE M, JØRGENSEN MH, et al. Second-line and third-line therapy for autoimmune hepatitis: A position statement from the European Reference Network on Hepatological Diseases and the International Autoimmune Hepatitis Group[J]. J Hepatol, 2020, 73( 6): 1496- 1506. DOI: 10.1016/j.jhep.2020.07.023. [4] FRIEDENSTEIN AJ. Precursor cells of mechanocytes[J]. Int Rev Cytol, 1976, 47: 327- 359. DOI: 10.1016/s0074-7696(08)60092-3. [5] NOORT WA, KRUISSELBRINK AB, IN'T ANKER PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD/SCID mice[J]. Exp Hematol, 2002, 30( 8): 870- 878. DOI: 10.1016/S0301-472X(02)00820-2. [6] di NICOLA M, CARLO-STELLA C, MAGNI M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli[J]. Blood, 2002, 99( 10): 3838- 3843. DOI: 10.1182/blood.v99.10.3838. [7] TSE WT, PENDLETON JD, BEYER WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation[J]. Transplantation, 2003, 75( 3): 389- 397. DOI: 10.1097/01.TP.0000045055.63901.A9. [8] CORCIONE A, BENVENUTO F, FERRETTI E, et al. Human mesenchymal stem cells modulate B-cell functions[J]. Blood, 2006, 107( 1): 367- 372. DOI: 10.1182/blood-2005-07-2657. [9] JIANG XX, ZHANG Y, LIU B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells[J]. Blood, 2005, 105( 10): 4120- 4126. DOI: 10.1182/blood-2004-02-0586. [10] ENGLISH K, BARRY FP, MAHON BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation[J]. Immunol Lett, 2008, 115( 1): 50- 58. DOI: 10.1016/j.imlet.2007.10.002. [11] SPAGGIARI GM, CAPOBIANCO A, BECCHETTI S, et al. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation[J]. Blood, 2006, 107( 4): 1484- 1490. DOI: 10.1182/blood-2005-07-2775. [12] SHI YF, WANG Y, LI Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases[J]. Nat Rev Nephrol, 2018, 14( 8): 493- 507. DOI: 10.1038/s41581-018-0023-5. [13] STEFAŃSKA K, VOLPONI AA, KULUS M, et al. Dental pulp stem cells-A basic research and future application in regenerative medicine[J]. Biomed Pharmacother, 2024, 178: 116990. DOI: 10.1016/j.biopha.2024.116990. [14] LIU H, GRONTHOS S, SHI S. Dental pulp stem cells[J]. Methods Enzymol, 2006, 419: 99- 113. DOI: 10.1016/s0076-6879(06)19005-9. [15] TAŞLı PN, TAPŞıN S, DEMIREL S, et al. Isolation and characterization of dental pulp stem cells from a patient with Papillon-Lefèvre syndrome[J]. J Endod, 2013, 39( 1): 31- 38. DOI: 10.1016/j.joen.2012.09.024. [16] RODAS-JUNCO BA, VILLICAÑA C. Dental pulp stem cells: Current advances in isolation, expansion and preservation[J]. Tissue Eng Regen Med, 2017, 14( 4): 333- 347. DOI: 10.1007/s13770-017-0036-3. [17] LAN XY, SUN ZW, CHU CY, et al. Dental pulp stem cells: An attractive alternative for cell therapy in ischemic stroke[J]. Front Neurol, 2019, 10: 824. DOI: 10.3389/fneur.2019.00824. [18] MATSUBARA K, MATSUSHITA Y, SAKAI K, et al. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity[J]. J Neurosci, 2015, 35( 6): 2452- 2464. DOI: 10.1523/JNEUROSCI.4088-14.2015. [19] LI FY, WANG XX, SHI J, et al. Anti-inflammatory effect of dental pulp stem cells[J]. Front Immunol, 2023, 14: 1284868. DOI: 10.3389/fimmu.2023.1284868. [20] DEMIRCAN PC, SARIBOYACI AE, UNAL ZS, et al. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: Comparison of transwell co-culture and mixed lymphocyte reaction systems[J]. Cytotherapy, 2011, 13( 10): 1205- 1220. DOI: 10.3109/14653249.2011.605351. [21] CHEN PX, LIN YC, LIN WB, et al. Human dental pulp stem cells have comparable abilities to umbilical cord mesenchymal stem/stromal cells in regulating inflammation and ameliorating hepatic fibrosis[J]. Hum Cell, 2024, 37( 1): 204- 213. DOI: 10.1007/s13577-023-01004-3. [22] IWANAKA T, YAMAZA T, SONODA S, et al. A model study for the manufacture and validation of clinical-grade deciduous dental pulp stem cells for chronic liver fibrosis treatment[J]. Stem Cell Res Ther, 2020, 11( 1): 134. DOI: 10.1186/s13287-020-01630-w. [23] LI Y, SONG GY, JIANG Y, et al. Single-cell transcriptome analysis of stem cells from human exfoliated deciduous teeth investigating functional heterogeneity in immunomodulation[J]. Sci Rep, 2024, 14( 1): 31279. DOI: 10.1038/s41598-024-82734-8. -



 PDF下载 ( 6205 KB)
PDF下载 ( 6205 KB)


 下载:
下载: