组织驻留记忆T细胞在慢性肝病中的调控机制与治疗靶点
DOI: 10.12449/JCH250526
The regulatory role of tissue-resident memory T cells in chronic liver diseases and associated therapeutic targets
-
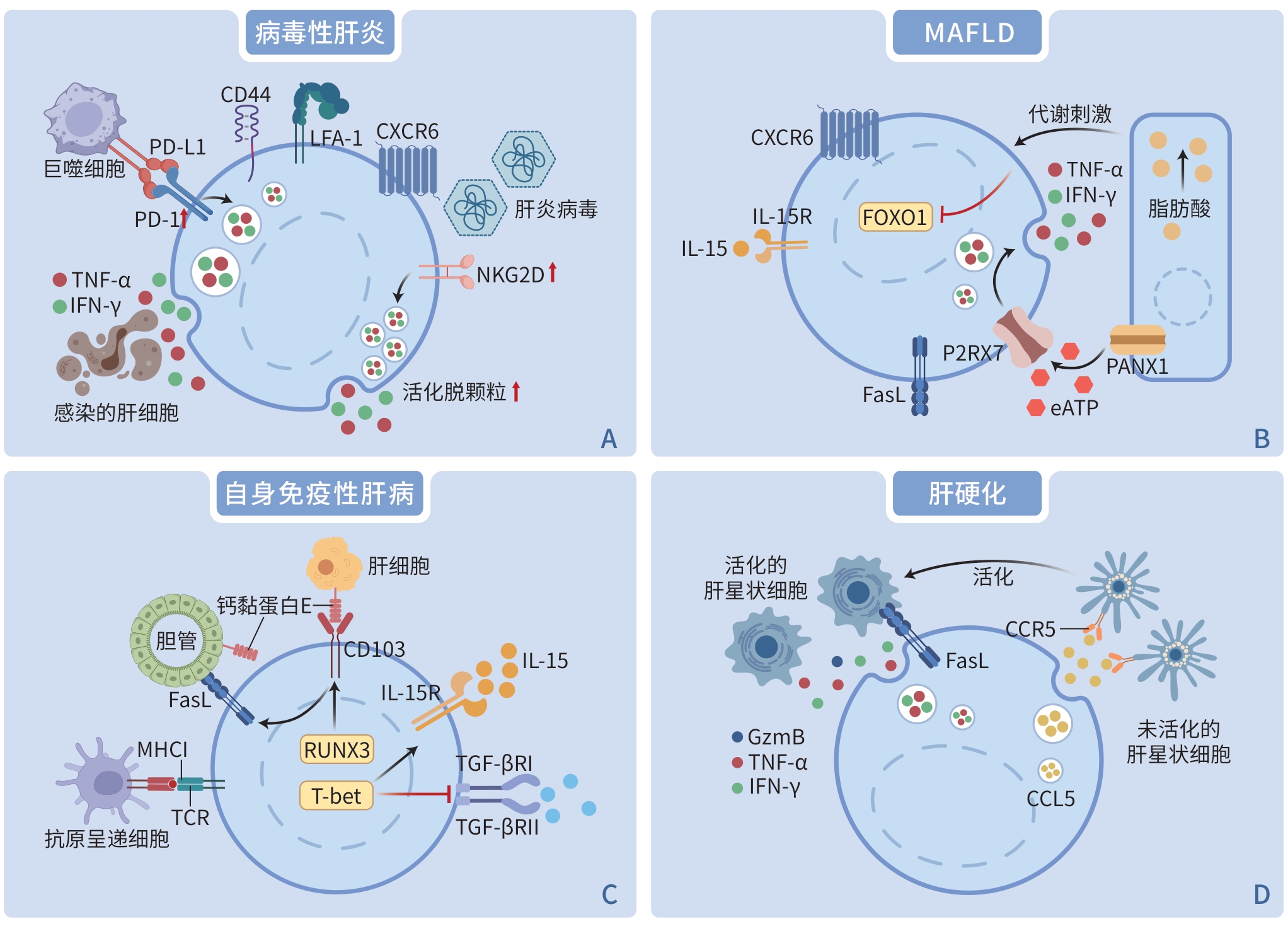
摘要: 组织驻留记忆T细胞(TRM细胞)是一类存在于组织中,具有组织特异性且不参与循环的记忆T细胞亚群。当潜在的危险攻击肝脏,如病原体(细菌或病毒等)侵袭或自身免疫反应过强时,TRM细胞作为第一道免疫防线,在病毒性肝炎、自身免疫性肝病、代谢相关脂肪性肝病、肝硬化和肝移植中都发挥着重要作用。本文阐述了肝脏TRM细胞的免疫表型,包括其表面标志物和转录谱,旨在进一步研究肝脏TRM细胞在慢性肝病中的作用,并探索其作为免疫治疗靶点的潜在功能。Abstract: Tissue-resident memory T cells (TRM cells) are a subset of memory T cells that reside in tissues, exhibit tissue specificity, and do not recirculate. When potential hazards threaten the liver, such as pathogen invasion (bacteria, viruses, etc.) and excessive autoimmune responses, TRM cells are essential as the first line of immune defense, playing an important role in viral hepatitis, autoimmune liver disease, metabolic dysfunction-associated fatty liver disease, liver cirrhosis, and liver transplantation. Here, we present the immunophenotypes of TRM cells in the liver and their surface markers and transcriptional profiles, aiming to clarify the role of TRM cells in chronic liver diseases and explore their potential function as therapeutic targets in immunotherapy.
-
Key words:
- Tissue-resident Memory T Cells /
- Liver Diseases /
- Immunotherapy
-
表 1 TRM 细胞的一般特征
Table 1. General features of TRM cells
项目 效应分子 功能 表面标志物 CD69 促进肝脏TRM 细胞的驻留状态并抑制S1PR1介导的细胞离开组织的过程 CD103 与钙黏蛋白E的结合可能对TRM细胞的定位、黏附和保留至关重要 CD49a 通过与整合素β1结合形成异二聚体VLA-1,促进整合素β1与胶原蛋白Ⅳ结合,导致驻留细胞保留在上皮细胞 转录谱 BLIMP1、HOBIT HOBIT和BLIMP1的共表达下调TRM细胞中CCR7、KLF-2及S1PR1的表达,促进细胞驻留在组织中 RUNX3 下调与循环T细胞发育相关的基因,并上调细胞驻留相关分子 BHLHE40 诱导应激反应蛋白的表达,以促进应激下TRM细胞的效应功能 TBX21(T-bet) 有助于IL-15受体的表达,以实现TRM细胞的长期存在 趋化因子 TRM 细胞的维持和效应功能已被证明需要持续的趋化因子刺激 趋化因子受体 CXCR3、CXCR6 通过结合单核细胞、肝窦内皮细胞和成纤维细胞分泌的多种趋化因子(如CXCL9、CXCL10、CXCL11和CXCL16),影响对TRM细胞的保留和维持 注:BLIMP1,B细胞诱导成熟蛋白1;HOBIT,T细胞中BLIMP1的同源物;RUNX3,RUNT相关转录因子3;BHLHE40,E类基本螺旋环螺旋蛋白40;TBX21(T-bet),T盒转录因子21;CXCR,CXC趋化因子受体;CXCL,趋化因子CXC配体。
-
[1] SALLUSTO F, LENIG D, FÖRSTER R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions[J]. Nature, 1999, 401( 6754): 708- 712. DOI: 10.1038/44385. [2] GEBHARDT T, WAKIM LM, EIDSMO L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus[J]. Nat Immunol, 2009, 10( 5): 524- 530. DOI: 10.1038/ni.1718. [3] MACKAY LK, RAHIMPOUR A, MA JZ, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin[J]. Nat Immunol, 2013, 14( 12): 1294- 1301. DOI: 10.1038/ni.2744. [4] WANG N, WANG YH, JIANG FL, et al. Research progress on differentiation and regulation of memory T cell subsets[J]. Chin J Immun, 2023, 39( 6): 1326- 1330, 1336. DOI: 10.3969/j.issn.1000-484X.2023.06.044.王宁, 王一晗, 姜凤良, 等. 记忆性T细胞亚群及其分化调控研究进展[J]. 中国免疫学杂志, 2023, 39( 6): 1326- 1330, 1336. DOI: 10.3969/j.issn.1000-484X.2023.06.044. [5] KUMAR BV, MA WJ, MIRON M, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites[J]. Cell Rep, 2017, 20( 12): 2921- 2934. DOI: 10.1016/j.celrep.2017.08.078. [6] WIJEYESINGHE S, BEURA LK, PIERSON MJ, et al. Expansible residence decentralizes immune homeostasis[J]. Nature, 2021, 592( 7854): 457- 462. DOI: 10.1038/s41586-021-03351-3. [7] SKON CN, LEE JY, ANDERSON KG, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells[J]. Nat Immunol, 2013, 14( 12): 1285- 1293. DOI: 10.1038/ni.2745. [8] PALLETT LJ, DAVIES J, COLBECK EJ, et al. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection[J]. J Exp Med, 2017, 214( 6): 1567- 1580. DOI: 10.1084/jem.20162115. [9] YOU ZR, LI Y, WANG QX, et al. The clinical significance of hepatic CD69+ CD103+ CD8+ resident-memory T cells in autoimmune hepatitis[J]. Hepatology, 2021, 74( 2): 847- 863. DOI: 10.1002/hep.31739. [10] RAY SJ, FRANKI SN, PIERCE RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection[J]. Immunity, 2004, 20( 2): 167- 179. DOI: 10.1016/s1074-7613(04)00021-4. [11] GRIFFITH JW, SOKOL CL, LUSTER AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity[J]. Annu Rev Immunol, 2014, 32: 659- 702. DOI: 10.1146/annurev-immunol-032713-120145. [12] FERNANDEZ-RUIZ D, NG WY, HOLZ LE, et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection[J]. Immunity, 2019, 51( 4): 780. DOI: 10.1016/j.immuni.2019.09.019. [13] KIM JH, HAN JW, CHOI YJ, et al. Functions of human liver CD69+CD103-CD8+ T cells depend on HIF-2α activity in healthy and pathologic livers[J]. J Hepatol, 2020, 72( 6): 1170- 1181. DOI: 10.1016/j.jhep.2020.01.010. [14] BUQUICCHIO FA, FONSECA R, YAN PK, et al. Distinct epigenomic landscapes underlie tissue-specific memory T cell differentiation[J]. Immunity, 2024, 57( 9): 2202- 2215. DOI: 10.1016/j.immuni.2024.06.014. [15] SOWELL RT, MARZO AL. Resident-memory CD8 T cells and mTOR: Generation, protection, and clinical importance[J]. Front Immunol, 2015, 6: 38. DOI: 10.3389/fimmu.2015.00038. [16] FRIZZELL H, FONSECA R, CHRISTO SN, et al. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes[J]. Sci Immunol, 2020, 5( 46): eaay9283. DOI: 10.1126/sciimmunol.aay9283. [17] PALLETT LJ, BURTON AR, AMIN OE, et al. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes[J]. J Exp Med, 2020, 217( 9): e20200050. DOI: 10.1084/jem.20200050. [18] WU LL, DENG H, FENG X, et al. Interferon-γ+ Th1 activates intrahepatic resident memory T cells to promote HBsAg loss by inducing M1 macrophage polarization[J]. J Med Virol, 2024, 96( 5): e29627. DOI: 10.1002/jmv.29627. [19] SUNG CC, HORNG JH, SIAO SH, et al. Asialo GM1-positive liver-resident CD8 T cells that express CD44 and LFA-1 are essential for immune clearance of hepatitis B virus[J]. Cell Mol Immunol, 2021, 18( 7): 1772- 1782. DOI: 10.1038/s41423-020-0376-0. [20] POCH T, KRAUSE J, CASAR C, et al. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis[J]. J Hepatol, 2021, 75( 2): 414- 423. DOI: 10.1016/j.jhep.2021.03.016. [21] TONNERRE P, WOLSKI D, SUBUDHI S, et al. Differentiation of exhausted CD8+ T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory[J]. Nat Immunol, 2021, 22( 8): 1030- 1041. DOI: 10.1038/s41590-021-00982-6. [22] KEFALAKES H, HORGAN XJ, JUNG MK, et al. Liver-resident bystander CD8+ T cells contribute to liver disease pathogenesis in chronic hepatitis D virus infection[J]. Gastroenterology, 2021, 161( 5): 1567- 1583. DOI: 10.1053/j.gastro.2021.07.027. [23] DUDEK M, PFISTER D, DONAKONDA S, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH[J]. Nature, 2021, 592( 7854): 444- 449. DOI: 10.1038/s41586-021-03233-8. [24] MARINOVIĆ S, LENARTIĆ M, MLADENIĆ K, et al. NKG2D-mediated detection of metabolically stressed hepatocytes by innate-like T cells is essential for initiation of NASH and fibrosis[J]. Sci Immunol, 2023, 8( 87): eadd1599. DOI: 10.1126/sciimmunol.add1599. [25] KODA Y, TERATANI T, CHU PS, et al. CD8+ tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells[J]. Nat Commun, 2021, 12: 4474. DOI: 10.1038/s41467-021-24734-0. [26] TRIVEDI PJ, HIRSCHFIELD GM. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases[J]. Gut, 2021, 70( 10): 1989- 2003. DOI: 10.1136/gutjnl-2020-322362. [27] ZIMMER CL, VON SETH E, BUGGERT M, et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells[J]. Sci Transl Med, 2021, 13( 599): eabb3107. DOI: 10.1126/scitranslmed.abb3107. [28] ZHU HX, YANG SH, GAO CY, et al. Targeting pathogenic CD8+ tissue-resident T cells with chimeric antigen receptor therapy in murine autoimmune cholangitis[J]. Nat Commun, 2024, 15: 2936. DOI: 10.1038/s41467-024-46654-5. [29] LI YK, LI B, XIAO X, et al. Itaconate inhibits CD103+ TRM cells and alleviates hepatobiliary injury in mouse models of primary sclerosing cholangitis[J]. Hepatology, 2024, 79( 1): 25- 38. DOI: 10.1097/HEP.0000000000000549. [30] FU JN, SYKES M. Emerging concepts of tissue-resident memory T cells in transplantation[J]. Transplantation, 2022, 106( 6): 1132- 1142. DOI: 10.1097/TP.0000000000004000. [31] LI XQ, LI SP, WANG Y, et al. Single cell RNA-sequencing delineates CD8+ tissue resident memory T cells maintaining rejection in liver transplantation[J]. Theranostics, 2024, 14( 12): 4844- 4860. DOI: 10.7150/thno.96928. [32] SCHARPING NE, RIVADENEIRA DB, MENK AV, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion[J]. Nat Immunol, 2021, 22( 2): 205- 215. DOI: 10.1038/s41590-020-00834-9. [33] PAN YD, TIAN T, PARK CO, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism[J]. Nature, 2017, 543( 7644): 252- 256. DOI: 10.1038/nature21379. [34] HUANG BY, LYU ZW, QIAN QW, et al. NUDT1 promotes the accumulation and longevity of CD103+ TRM cells in primary biliary cholangitis[J]. J Hepatol, 2022, 77( 5): 1311- 1324. DOI: 10.1016/j.jhep.2022.06.014. [35] TSUZUKI T, NAKATSU Y, NAKABEPPU Y. Significance of error-avoiding mechanisms for oxidative DNA damage in carcinogenesis[J]. Cancer Sci, 2007, 98( 4): 465- 470. DOI: 10.1111/j.1349-7006.2007.00409.x. [36] MARIATHASAN S, WEISS DS, NEWTON K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP[J]. Nature, 2006, 440( 7081): 228- 232. DOI: 10.1038/nature04515. [37] BORGES DA SILVA H, BEURA LK, WANG HG, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells[J]. Nature, 2018, 559( 7713): 264- 268. DOI: 10.1038/s41586-018-0282-0. [38] CAIN DW, CIDLOWSKI JA. Immune regulation by glucocorticoids[J]. Nat Rev Immunol, 2017, 17( 4): 233- 247. DOI: 10.1038/nri.2017.1. [39] MILNER JJ, TOMA C, YU B, et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours[J]. Nature, 2017, 552( 7684): 253- 257. DOI: 10.1038/nature24993. [40] SWADLING L, PALLETT LJ, DINIZ MO, et al. Human liver memory CD8+ T cells use autophagy for tissue residence[J]. Cell Rep, 2020, 30( 3): 687- 698. DOI: 10.1016/j.celrep.2019.12.050. [41] WIGGINS BG, PALLETT LJ, LI XY, et al. The human liver microenvironment shapes the homing and function of CD4+ T-cell populations[J]. Gut, 2022, 71( 7): 1399- 1411. DOI: 10.1136/gutjnl-2020-323771. [42] FONSECA R, BURN TN, GANDOLFO LC, et al. Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells[J]. Nat Immunol, 2022, 23( 8): 1236- 1245. DOI: 10.1038/s41590-022-01273-4. [43] CHEN CJ, YIN Y, SHI GN, et al. A highly selective JAK3 inhibitor is developed for treating rheumatoid arthritis by suppressing γc cytokine-related JAK-STAT signal[J]. Sci Adv, 2022, 8( 33): eabo4363. DOI: 10.1126/sciadv.abo4363. [44] MERAVIGLIA-CRIVELLI D, VILLANUEVA H, ZHELEVA A, et al. IL-6/STAT3 signaling in tumor cells restricts the expression of frameshift-derived neoantigens by SMG1 induction[J]. Mol Cancer, 2022, 21: 211. DOI: 10.1186/s12943-022-01679-6. [45] ZHOU P, TAO K, ZENG L, et al. IRG1/Itaconate inhibits proliferation and promotes apoptosis of CD69+CD103+CD8+ tissue-resident memory T cells in autoimmune hepatitis by regulating the JAK3/STAT3/P53 signalling pathway[J]. Apoptosis, 2024, 29( 9-10): 1738- 1756. DOI: 10.1007/s10495-024-01970-5. [46] LI C, HE YY, ZHANG YT, et al. Tauroursodeoxycholic acid(TUDCA) disparate pharmacological effects to lung tissue-resident memory T cells contribute to alleviated silicosis[J]. Biomed Pharmacother, 2022, 151: 113173. DOI: 10.1016/j.biopha.2022.113173. [47] RAKHRA K, ABRAHAM W, WANG CS, et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells[J]. Sci Immunol, 2021, 6( 57): eabd8003. DOI: 10.1126/sciimmunol.abd8003. -



 PDF下载 ( 1694 KB)
PDF下载 ( 1694 KB)


 下载:
下载: