胸腺基质淋巴细胞生成素在对乙酰氨基酚诱导的急性肝损伤小鼠模型中的作用机制
DOI: 10.12449/JCH250117
伦理学声明: 本研究方案于2021年2月1日经由广西医科大学动物实验中心实验动物伦理委员会审批,批号:202005022,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:陈文赏负责课题设计,资料分析,撰写论文;尹明景参与收集数据,修改论文;朱继金负责拟定写作思路,指导撰写文章并最后定稿。
Mechanism of action of thymic stromal lymphopoietin in a mouse model of acetaminophen-induced acute liver injury
-
摘要:
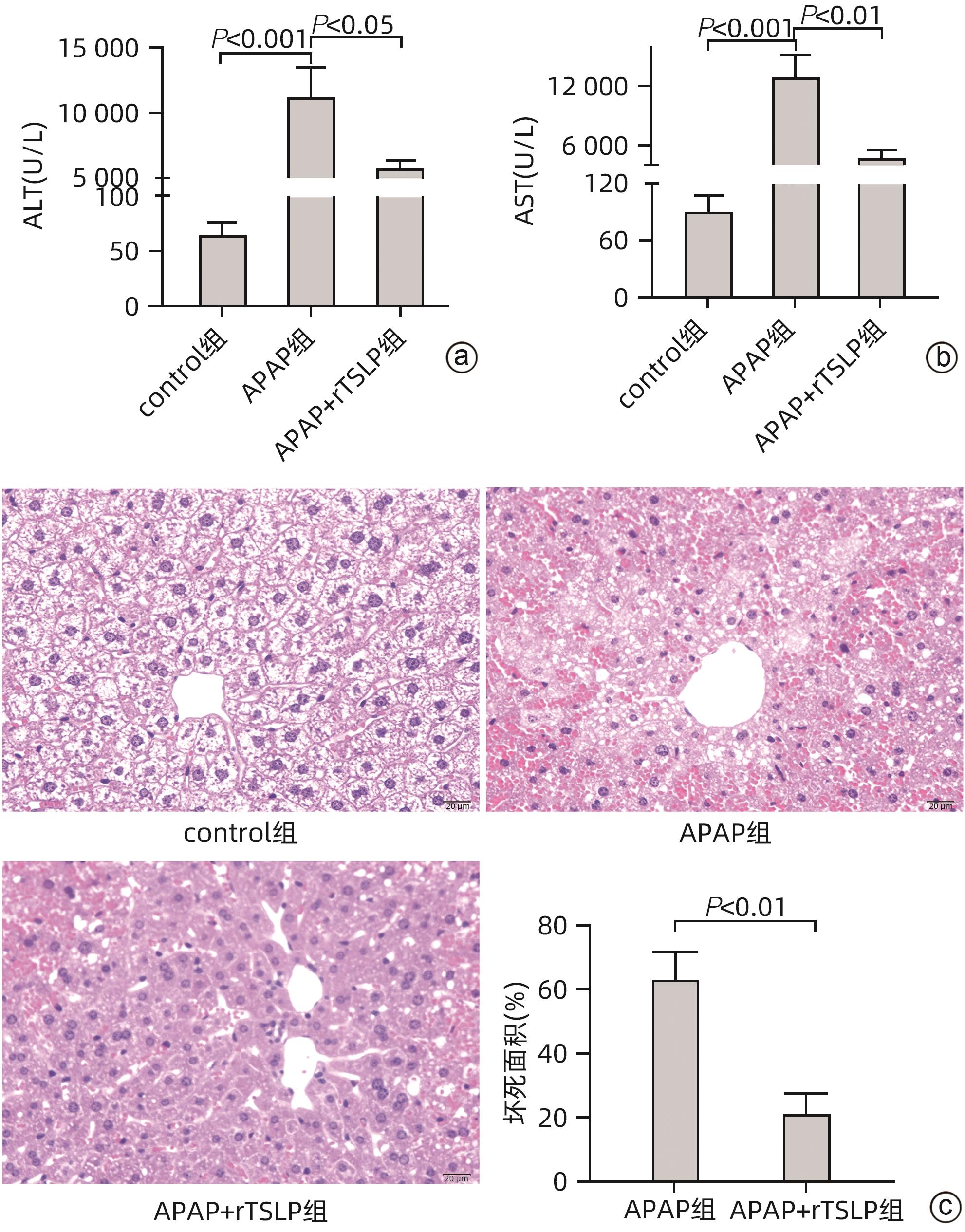
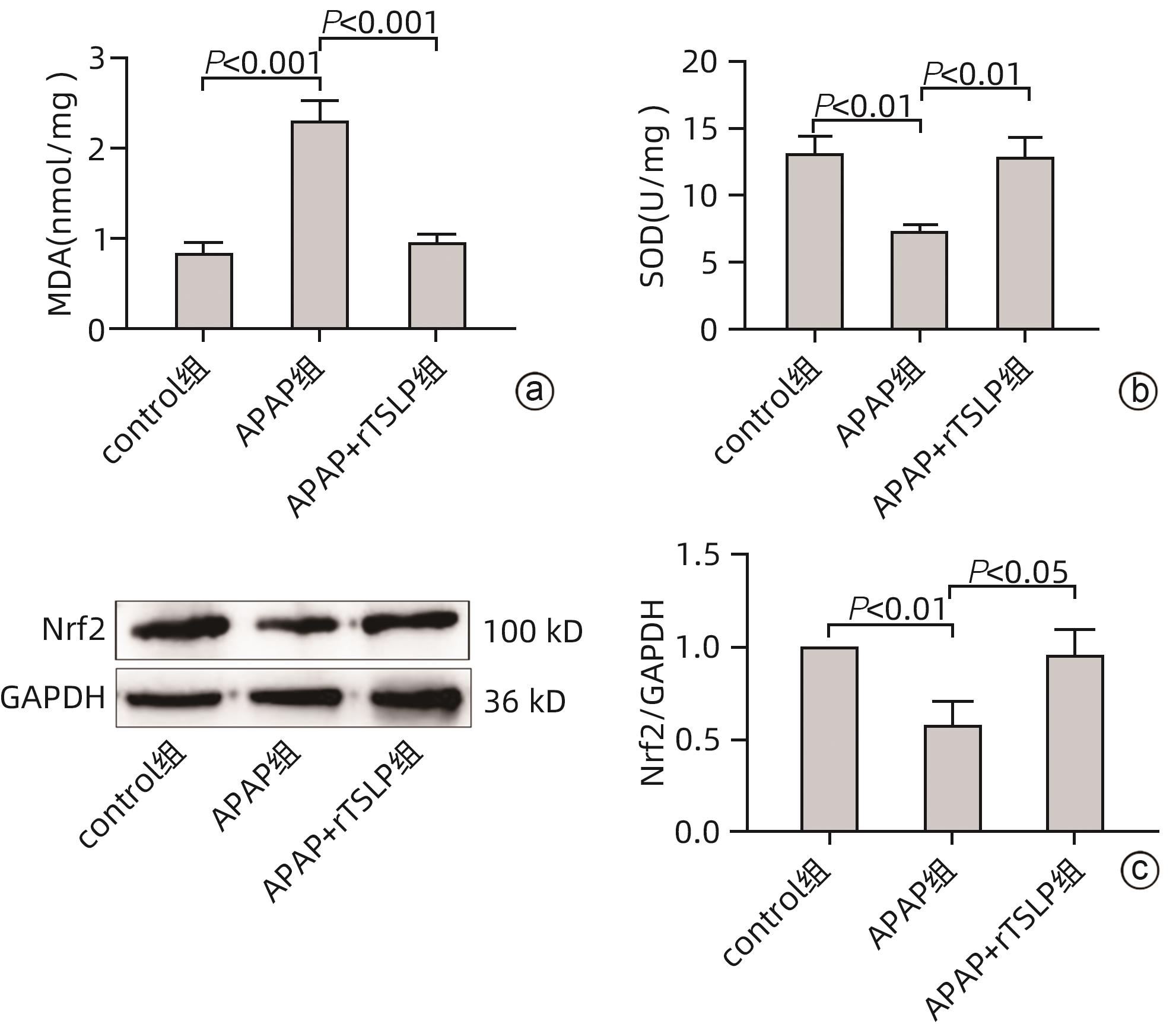
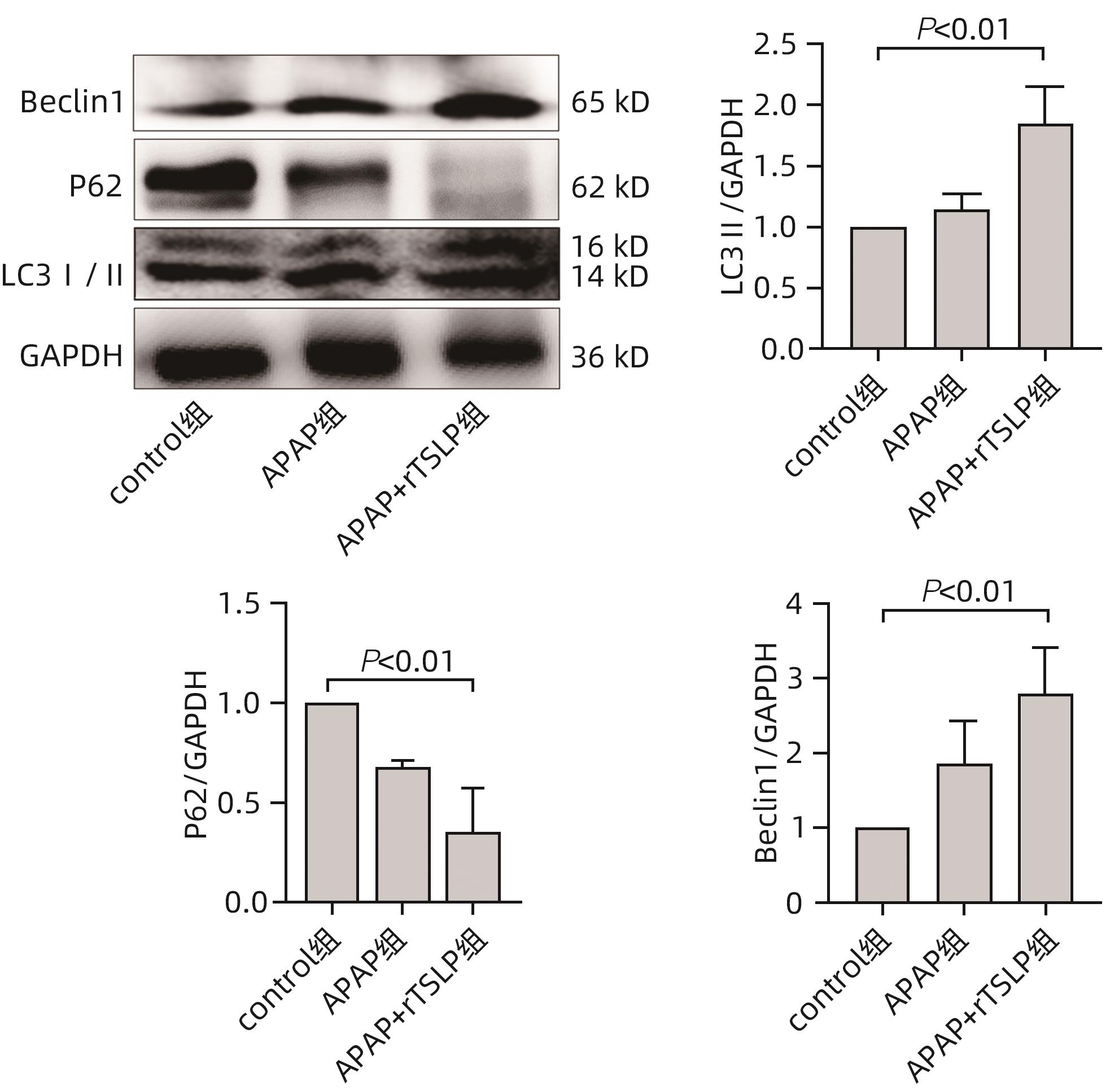
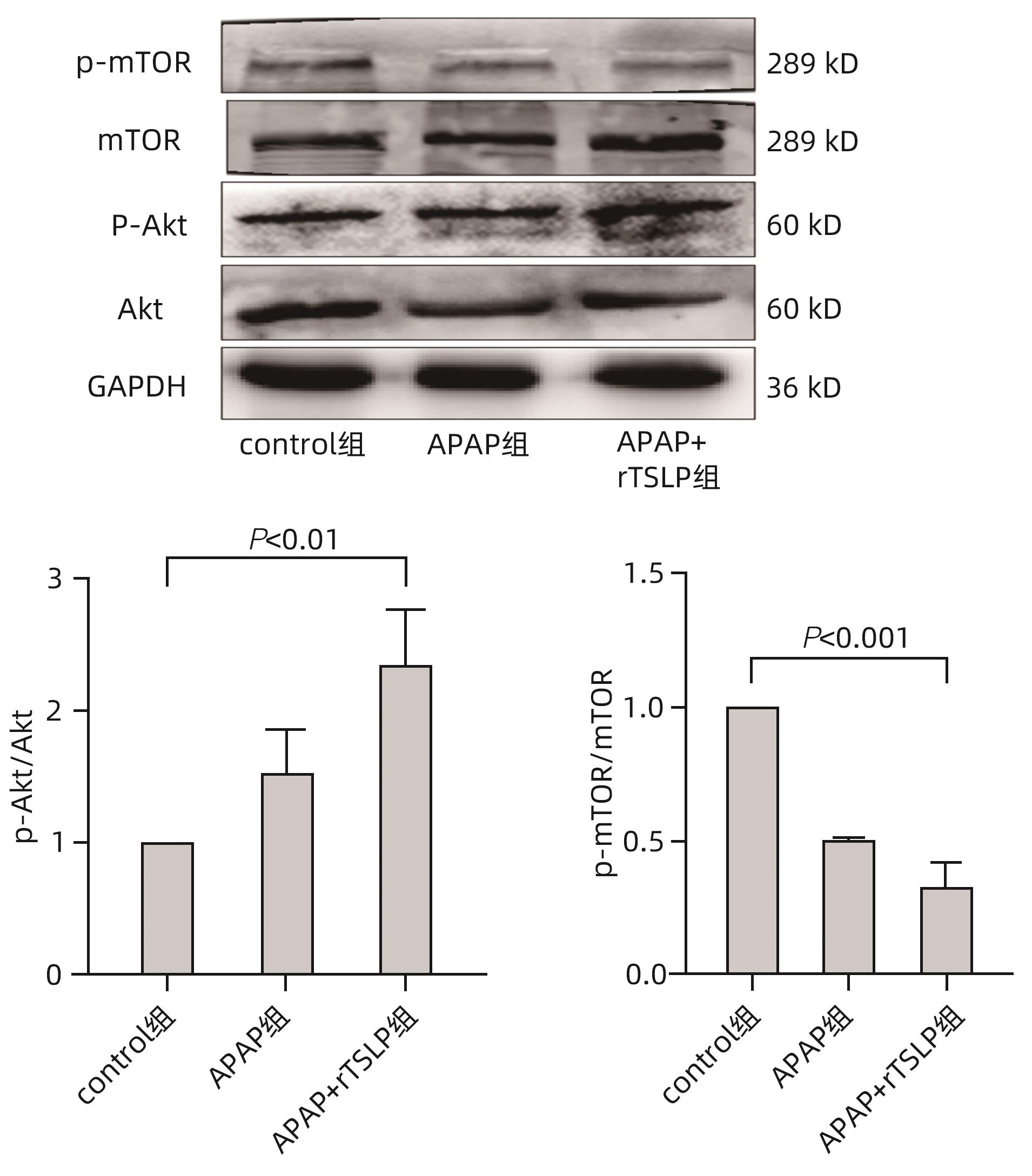
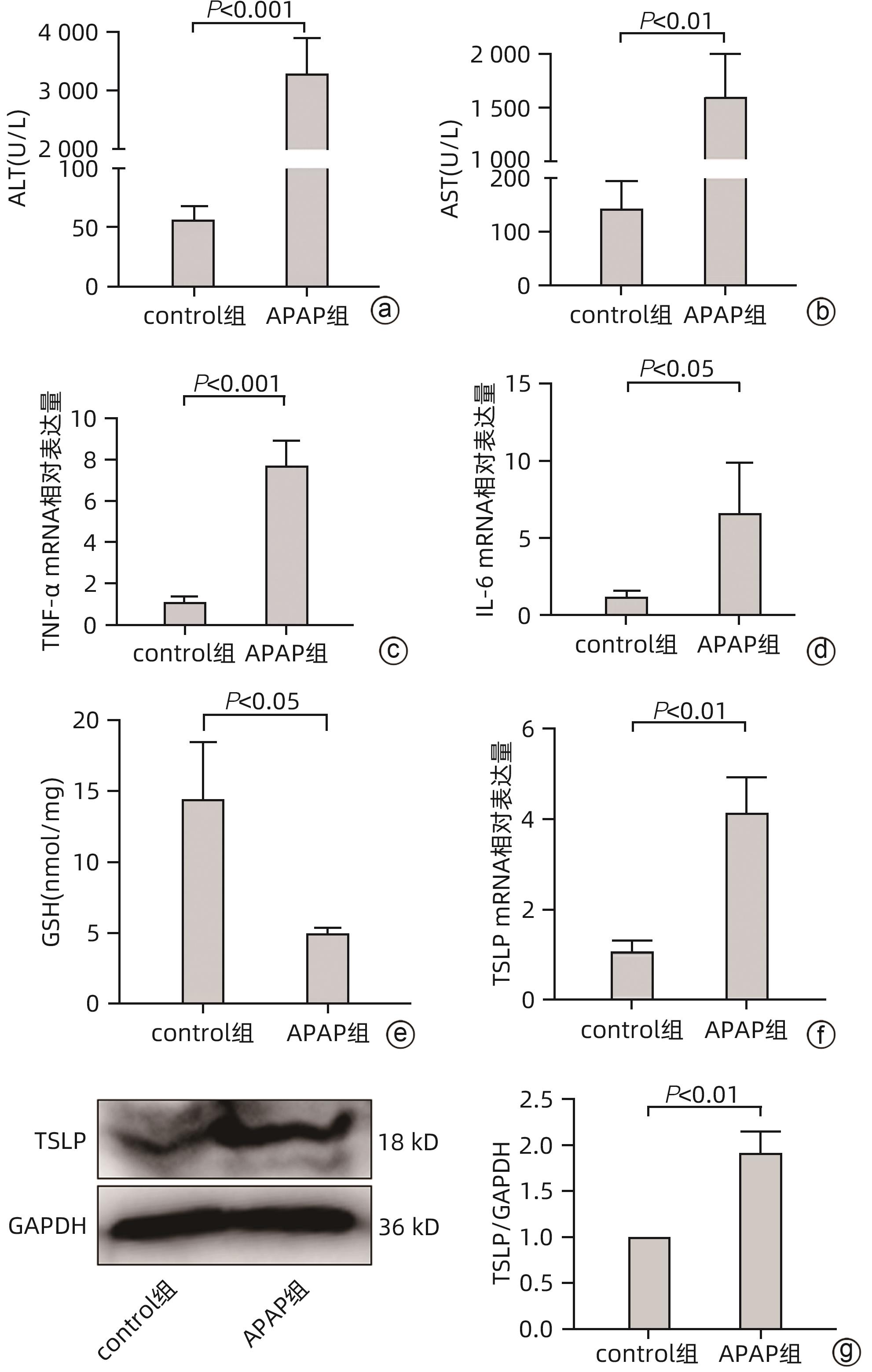
目的 探讨胸腺基质淋巴细胞生成素(TSLP)在对乙酰氨基酚(APAP)诱导的急性肝损伤小鼠模型中的作用及其机制。 方法 16只野生型(WT)雄性C57BL/6J小鼠被随机分为2组,分别为control组和APAP组,每组8只;APAP组按照400 mg/kg的剂量腹腔注射APAP溶液建模,control组注射等体积生理盐水,6 h后进行取材。全自动化学分析仪检测血清ALT及AST,实时定量PCR方法检测肝组织炎症因子TNF-α和IL-6的mRNA表达,试剂盒检测肝组织匀浆中谷胱甘肽(GSH)含量,实时定量PCR、Western Blot方法检测TSLP的转录和蛋白水平的表达。另取22只WT雄性C57BL/6J小鼠,随机分为3组,分别为control组(n=8)、APAP组(n=8)和APAP+rTSLP组(n=6),APAP+rTSLP组先腹腔注射rTSLP溶液,同时control组、APAP组注射溶剂PBS;30 min后APAP+rTSLP组和APAP组注射APAP溶液,control组注射等体积生理盐水。检测3组小鼠血清ALT及AST;通过HE染色观察小鼠肝脏的病理变化;试剂盒检测肝组织匀浆中氧化应激指标丙二醛(MDA)、超氧化物歧化酶(SOD)水平;Western Blot方法检测自噬相关蛋白LC3Ⅰ/Ⅱ、Beclin1、P62,以及核因子E2相关因子2(Nrf2)、蛋白激酶B(Akt)、磷酸化-Akt(p-Akt)、哺乳动物雷帕霉素靶蛋白(mTOR)、磷酸化-mTOR(p-mTOR)等分子的蛋白表达。此外,取16只WT雄性C57BL/6J小鼠和16只沉默TSLP受体(TSLPR-/-)小鼠,分为WT小鼠control组、WT小鼠APAP组、TSLPR-/-小鼠control组和TSLPR-/-小鼠APAP组,每组8只,WT小鼠APAP组和TSLPR-/-小鼠APAP组按照400 mg/kg的剂量腹腔注射APAP溶液建模,WT小鼠control组和TSLPR-/-小鼠control组注射等体积生理盐水。检测4组小鼠血清ALT、AST以及肝组织的MDA含量;Western Blot方法检测LC3Ⅰ/Ⅱ、Akt、p-Akt的蛋白表达。计量资料两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 APAP诱导急性肝损伤小鼠建模成功后,肝脏TSLP的mRNA和蛋白表达水平较control组均升高(P值均<0.01)。在应用rTSLP的研究中,相比于control组,APAP组的ALT、AST明显升高(P值均<0.001),肝组织HE染色呈现沿中央静脉放射状坏死,氧化应激指标SOD、Nrf2蛋白表达下降,MDA水平上升(P值均<0.01);而APAP+rTSLP组较APAP组,ALT、AST下降,肝组织坏死面积减小,SOD、Nrf2蛋白表达升高,MDA下降(P值均<0.05);APAP+rTSLP组与control组相比,LC3Ⅰ/Ⅱ、Beclin1、P62、p-Akt、p-mTOR蛋白表达差异均有统计学意义(P值均<0.01)。在应用TSLPR-/-小鼠的研究中,建模后,TSLPR-/-小鼠相较于WT小鼠,ALT、AST、MDA升高,LC3Ⅰ/Ⅱ、p-Akt蛋白表达下降(P值均<0.01)。 结论 TSLP能够增加自噬,降低氧化应激,从而改善过量APAP引起的急性肝损伤,并且其作用机制可能与PI3K/Akt信号通路的激活和mTOR的抑制有关。 -
关键词:
- 胸腺基质淋巴细胞生成素 /
- 醋氨酚 /
- 化学性与药物性肝损伤 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the role and mechanism of thymic stromal lymphopoietin (TSLP) in a mouse model of acetaminophen (APAP)-induced acute liver injury. Methods A total of 16 wild-type (WT) male C57BL/6J mice were randomly divided into control group and APAP group, with 8 mice in each group, and the mice in the APAP group were given intraperitoneal injection of APAP solution at a dose of 400 mg/kg to establish an animal model, while those in the control group were given injection of an equal volume of normal saline, with samples collected after 6 hours. An automatic chemical analyzer was used to measure the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST); quantitative real-time PCR was used to measure the mRNA expression levels of the inflammatory factors tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in liver tissue; the kit was used to measure the content of glutathione (GSH) in liver tissue homogenate; quantitative real-time PCR and Western blot were used to measure the transcriptional level and protein expression level of TSLP. Furthermore, 22 WT male C57BL/6J mice were randomly divided into control group with 8 mice, APAP group with 8 mice, and APAP+recombination TSLP (rTSLP) group with 6 mice; the mice in the APAP+rTSLP group were given intraperitoneal injection of rTSLP solution, while those in the control group and the APAP group were given injection of the solvent PBS; after 30 minutes, the mice in the APAP+rTSLP group and the APAP group were given injection of APAP solution, while those in the control group were given injection of an equal volume of normal saline. The serum levels of ALT and AST were measured; HE staining was used to observe the pathological changes of the liver; kits were used to measure the levels of the oxidative stress indices malondialdehyde (MDA) and superoxide dismutase (SOD) in liver tissue homogenate; Western blot was used to measure the expression levels of the autophagy-related proteins LC3Ⅰ/Ⅱ, Beclin1, and P62 and the molecules such as nuclear factor erythroid 2-related factor 2 (Nrf2), protein kinase B (Akt), phosphorylated Akt (p-Akt), mammalian target of rapamycin (mTOR), and phosphorylated mTOR (p-mTOR). In addition, 16 WT male C57BL/6J mice and 16 TSLP receptor-silenced (TSLPR-/-) mice were divided into WT mouse control group, WT mouse APAP group, TSLPR-/- mouse control group, and TSLPR-/- mouse APAP group, with 8 mice in each group; the mice in the WT mouse APAP group and the TSLPR-/- mouse APAP group were used for modeling by intraperitoneal injection of APAP solution at a dose of 400 mg/kg, and those in the WT mouse control group and the TSLPR-/- mouse control group were given injection of an equal volume of normal saline. The serum levels of ALT and AST and the content of MDA in liver tissue were measured for these four groups, and Western blot was used to measure the protein expression levels of LC3Ⅰ/Ⅱ, Akt, and p-Akt. The independent-samples t test was used for comparison of continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results After the mouse model of APAP-induced acute liver injury was established successfully, there were significant increases in the mRNA and protein expression levels of TSLP compared with the control group (both P<0.01). In the study of rTSLP, compared with the control group, the APAP group had significant increases in ALT and AST (both P<0.001) and radial necrosis along the central vein observed by HE staining of liver tissue, as well as significant reductions in the protein expression levels of the oxidative stress indices SOD and Nrf2 and a significant increase in the level of MDA (all P<0.01); compared with the APAP group, the APAP+rTSLP group had significant reductions in ALT and AST, a significant reduction in necrotic area of liver tissue, significant increases in the protein expression levels of SOD and Nrf2, and a significant reduction in MDA (all P<0.05); there were significant differences in the protein expression levels of LC3Ⅰ/Ⅱ, Beclin1, P62, p-Akt, and p-mTOR between the APAP+rTSLP group and the control group (all P<0.01). In the study of TSLPR-/- mice, compared with the WT mice after modeling, the TSLPR-/- mice had significant increases in the levels of ALT, AST, and MDA and significant reductions in the expression levels of LC3Ⅰ/Ⅱ and p-Akt (all P<0.05). Conclusion TSLP can increase autophagy, reduce oxidative stress, and thus improve acute liver injury induced by APAP overdose, possibly by activating the PI3K/Akt signaling pathway and inhibiting mTOR. -
表 1 引物序列
Table 1. Primers sequence
引物名称 正向引物(5'-3') 反向引物(5'-3') GAPDH GACATGCCGCCTGGAGAAAC AGCCCAGGATGCCCTTTAGT TSLP CTCAATCCTATCCCTGGCT GACTTCTTGTGCCATTTCCT TNF-α ACGGCATGGATCTCAAAGAC AGATAGCAAATCGGCTGAGG IL-6 ACAACCACGGCCTTCCCTA TCCACGATTTCCCAGAGAACA -
[1] GROENEVELD D, CLINE-FEDEWA H, BAKER KS, et al. Von Willebrand factor delays liver repair after acetaminophen-induced acute liver injury in mice[J]. J Hepatol, 2020, 72( 1): 146- 155. DOI: 10.1016/j.jhep.2019.09.030. [2] ZHANG R, ZHANG F. Analysis of clinical misdiagnosis of drug⁃induced liver injury induced by acetaminophen[J]. Clin Misdiagn Misther, 2023, 36( 5): 15- 18. DOI: 10.3969/j.issn.1002-3429.2023.05.004.张蕊, 张芳. 对乙酰氨基酚致药物性肝损伤临床误诊分析[J]. 临床误诊误治, 2023, 36( 5): 15- 18. DOI: 10.3969/j.issn.1002-3429.2023. 05.004. [3] RAMACHANDRAN A, JAESCHKE H. A mitochondrial journey through acetaminophen hepatotoxicity[J]. Food Chem Toxicol, 2020, 140: 111282. DOI: 10.1016/j.fct.2020.111282. [4] CHEN WS, ZHU JJ, LI SL. C-Jun N-terminal kinase signaling pathway in acetaminophen-induced liver injury[J]. Chin Crit Care Med, 2023, 35( 11): 1223- 1228. DOI: 10.3760/cma.j.cn121430-20221205-01060.陈文赏, 朱继金, 李仕来. 对乙酰氨基酚致急性肝损伤中的c-Jun氨基末端激酶信号通路[J]. 中华危重病急救医学, 2023, 35( 11): 1223- 1228. DOI: 10.3760/cma.j.cn121430-20221205-01060. [5] HE R, GEHA RS. Thymic stromal lymphopoietin[J]. Ann N Y Acad Sci, 2010, 1183: 13- 24. DOI: 10.1111/j.1749-6632.2009.05128.x. [6] VARRICCHI G, PECORARO A, MARONE G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer[J]. Front Immunol, 2018, 9: 1595. DOI: 10.3389/fimmu.2018.01595. [7] CHEN WS, ZHU JJ, LI SL. Role of thymic stromal lymphopoietin in liver diseases[J]. J Clin Hepatol, 2022, 38( 5): 1175- 1178. DOI: 10.3969/j.issn.1001-5256.2022.05.042.陈文赏, 朱继金, 李仕来. 胸腺基质淋巴细胞生成素在肝脏疾病中的作用[J]. 临床肝胆病杂志, 2022, 38( 5): 1175- 1178. DOI: 10.3969/j.issn.1001-5256.2022.05.042. [8] LI SL, YI ZJ, DENG MH, et al. TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway[J]. JCI Insight, 2019, 4( 22): e129013. DOI: 10.1172/jci.insight.129013. [9] CHAO XJ, WANG H, JAESCHKE H, et al. Role and mechanisms of autophagy in acetaminophen-induced liver injury[J]. Liver Int, 2018, 38( 8): 1363- 1374. DOI: 10.1111/liv.13866. [10] YING GQ, ZHANG YL, TANG GQ, et al. Functions of thymic stromal lymphopoietin in non-allergic diseases[J]. Cell Immunol, 2015, 295( 2): 144- 149. DOI: 10.1016/j.cellimm.2015.03.006. [11] SANSONNO D, RUSSI S, SANSONNO S, et al. Thymic stromal lymphopoietin in hepatitis C virus-related cryoglobulinemic vasculitis: Gene expression level and protein distribution[J]. Arthritis Res Ther, 2015, 17( 1): 62. DOI: 10.1186/s13075-015-0581-x. [12] PROCTOR WR, CHAKRABORTY M, FULLERTON AM, et al. Thymic stromal lymphopoietin and interleukin-4 mediate the pathogenesis of halothane-induced liver injury in mice[J]. Hepatology, 2014, 60( 5): 1741- 1752. DOI: 10.1002/hep.27169. [13] ZHONG CC, ZHAO T, HOGSTRAND C, et al. Copper(Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways[J]. J Nutr Biochem, 2022, 100: 108883. DOI: 10.1016/j.jnutbio.2021.108883. [14] FAN XY, WANG LD, HUANG JB, et al. Pterostilbene reduces acetaminophen-induced liver injury by activating the Nrf2 antioxidative defense system via the AMPK/Akt/GSK3β pathway[J]. Cell Physiol Biochem, 2018, 49( 5): 1943- 1958. DOI: 10.1159/000493655. [15] CHAN K, HAN XD, KAN YW. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen[J]. Proc Natl Acad Sci U S A, 2001, 98( 8): 4611- 4616. DOI: 10.1073/pnas.081082098. [16] ENOMOTO A, ITOH K, NAGAYOSHI E, et al. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes[J]. Toxicol Sci, 2001, 59( 1): 169- 177. DOI: 10.1093/toxsci/59.1.169. [17] CHOWDHURY A, NABILA J, ADELUSI TEMITOPE I, et al. Current etiological comprehension and therapeutic targets of acetaminophen-induced hepatotoxicity[J]. Pharmacol Res, 2020, 161: 105102. DOI: 10.1016/j.phrs.2020.105102. [18] YI ZJ, DENG MH, SCOTT MJ, et al. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in hepatocytes to protect against liver ischemia-reperfusion injury[J]. Hepatology, 2020, 72( 4): 1394- 1411. DOI: 10.1002/hep.31147. [19] WANG XY, WEI ZQ, JIANG YF, et al. mTOR signaling: The interface linking cellular metabolism and hepatitis B virus replication[J]. Virol Sin, 2021, 36( 6): 1303- 1314. DOI: 10.1007/s12250-021-00450-3. [20] WANG Z, HAO WN, HU JN, et al. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways[J]. Antioxidants(Basel), 2019, 8( 9): 395. DOI: 10.3390/antiox8090395. [21] YANG HQ, WANG H, LIU YB, et al. The PI3K/Akt/mTOR signaling pathway plays a role in regulating aconitine-induced autophagy in mouse liver[J]. Res Vet Sci, 2019, 124: 317- 320. DOI: 10.1016/j.rvsc.2019.04.016. [22] XIU AY, DING Q, LI Z, et al. Doxazosin attenuates liver fibrosis by inhibiting autophagy in hepatic stellate cells via activation of the PI3K/Akt/mTOR signaling pathway[J]. Drug Des Devel Ther, 2021, 15: 3643- 3659. DOI: 10.2147/DDDT.S317701. [23] PALLIYAGURU DL, CHARTOUMPEKIS DV, WAKABAYASHI N, et al. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms[J]. Free Radic Biol Med, 2016, 101: 116- 128. DOI: 10.1016/j.freeradbiomed.2016.10.003. -



 PDF下载 ( 2533 KB)
PDF下载 ( 2533 KB)

 下载:
下载: