恩替卡韦与富马酸丙酚替诺福韦初治慢性乙型肝炎患者的肾功能变化比较及影响因素分析
DOI: 10.12449/JCH250107
Changes in renal function in chronic hepatitis B patients treated initially with entecavir versus tenofovir alafenamide fumarate and related influencing factors
-
摘要:
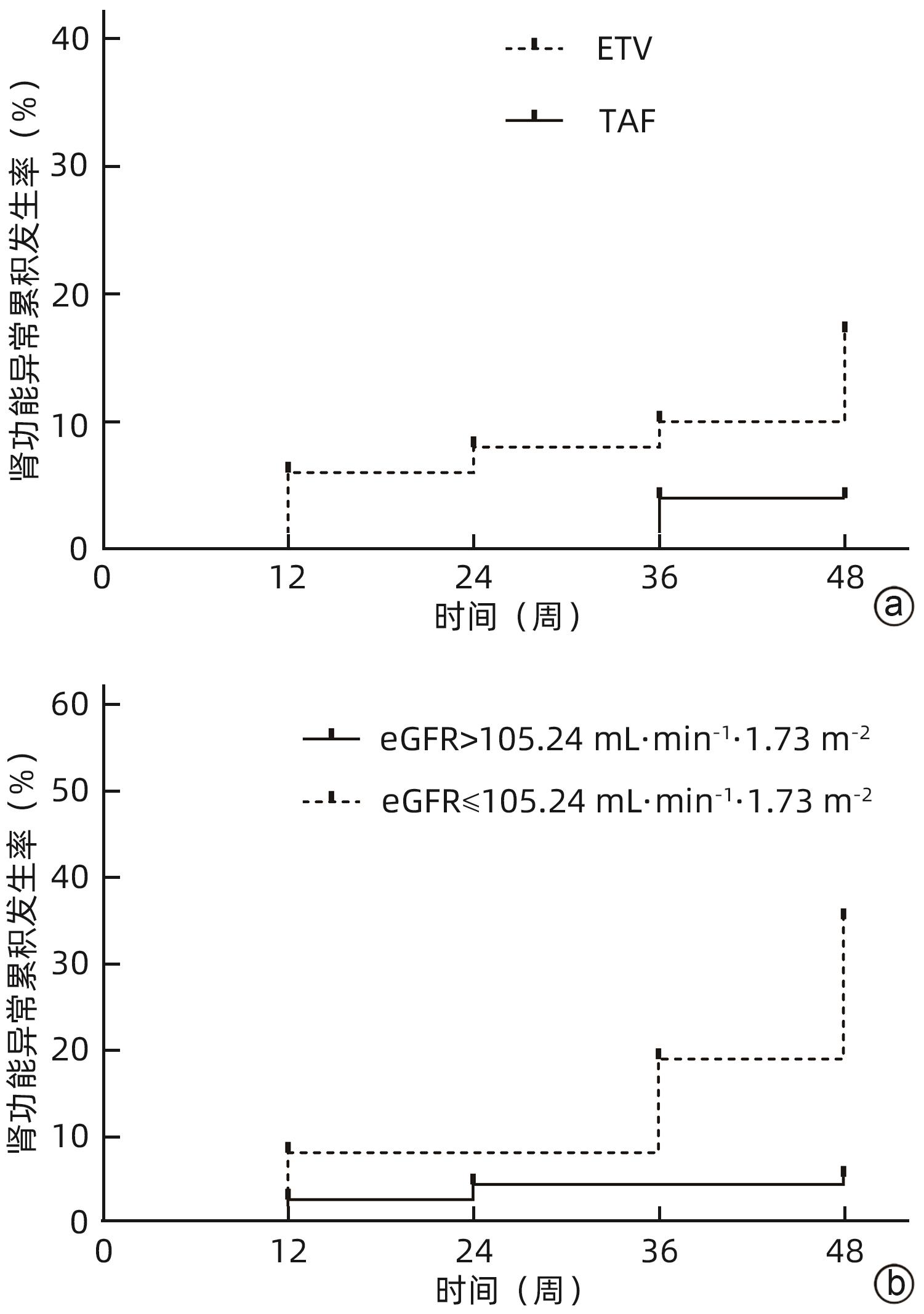
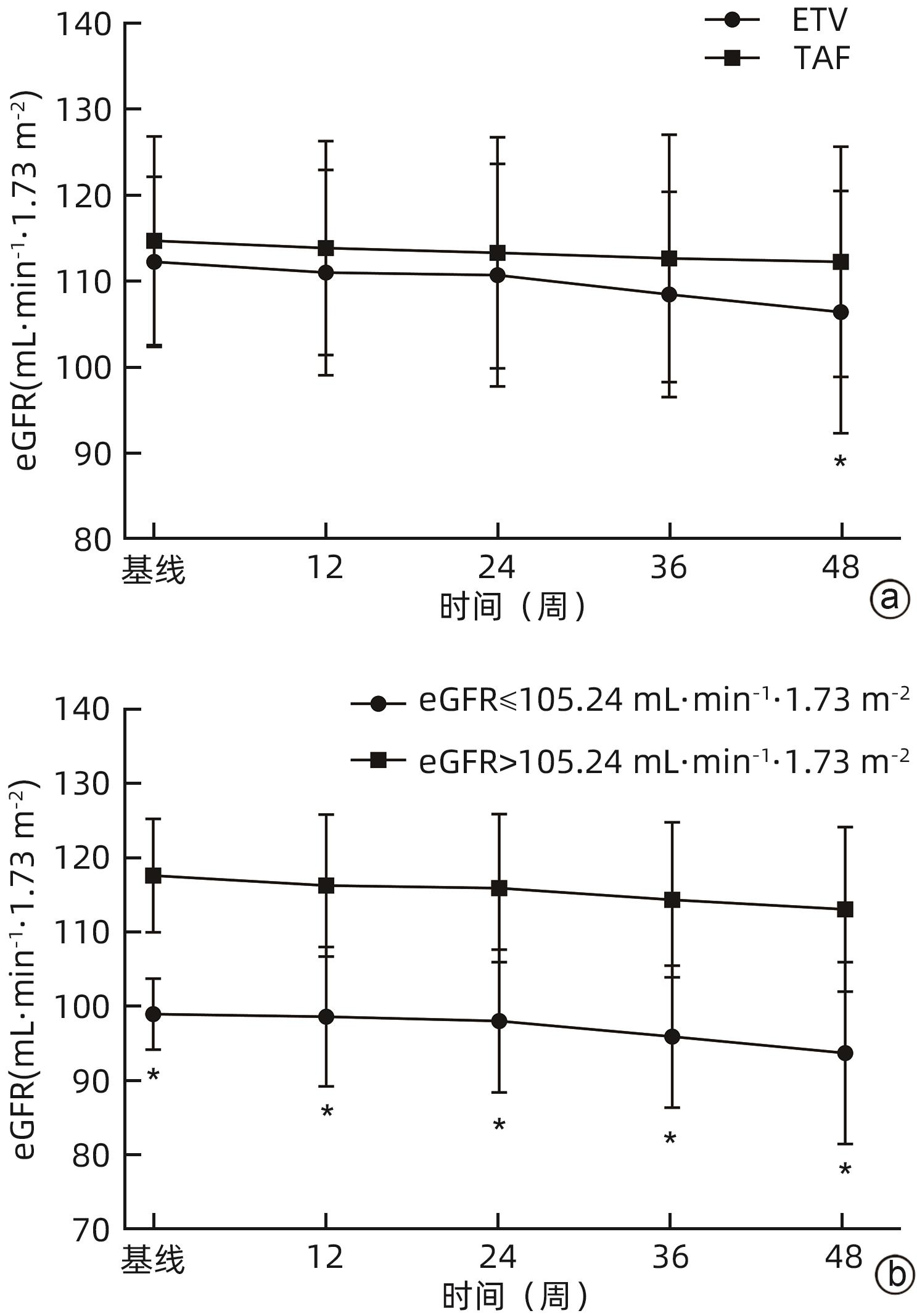
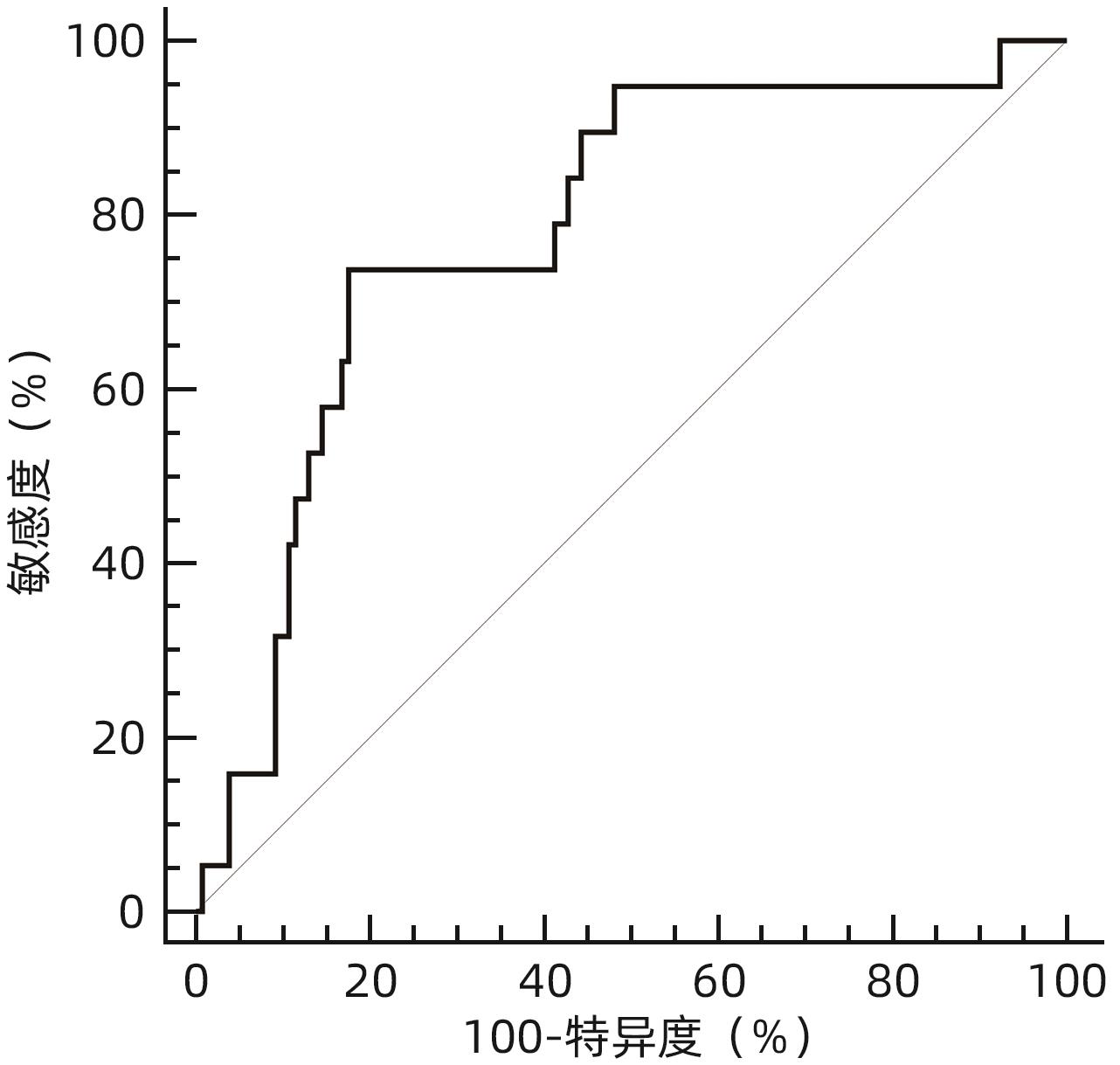
目的 分析比较恩替卡韦(ETV)与富马酸丙酚替诺福韦(TAF)对初治慢性乙型肝炎(CHB)患者肾功能的影响。 方法 回顾性分析2019年9月—2023年11月于南昌大学第一附属医院感染科门诊接受ETV或TAF治疗至少48周的167例初治CHB患者临床资料。根据患者抗病毒药物分为ETV组(n=117)和TAF组(n=50)。为均衡基线临床资料,采用倾向性评分匹配(PSM)按照2∶1比例进行匹配分析,比较两组患者48周时肾小球滤过率(eGFR)水平及肾功能异常发生率。根据患者48周eGFR水平将患者分为肾功能正常组和异常组。计量资料两组间比较采用成组t检验或Mann-Whitney U检验;计数资料组间比较采用χ2检验或Fisher精确检验。采用多因素Logistic回归分析发生肾功能异常的影响因素,受试者工作特征曲线(ROC曲线)评估各指标预测发生肾功能异常的效能。采用Kaplan-Meier法分析肾功能异常累积发生率,并应用Log-rank检验进行比较。采用重复测量资料的方差分析比较CHB患者抗病毒治疗期间eGFR动态变化情况。 结果 PSM成功匹配150例CHB患者,其中ETV组100例,TAF组50例。基线时,ETV组和TAF组一般资料比较,差异均无统计学意义(P值均>0.05),两组患者基线eGFR分别为(112.29±9.92) mL·min-1·1.73 m-2和(114.72±12.15) mL·min-1·1.73 m-2。48周时两组患者eGFR水平较基线均有下降,ETV组48周eGFR明显低于TAF组[(106.42±14.12) mL·min-1·1.73 m-2 vs (112.25±13.44) mL·min-1·1.73 m-2,t=-2.422,P=0.017],肾功能异常发生率明显高于TAF组(17.00% vs 4.00%,χ2=5.092,P=0.024)。将患者分为肾功能正常组(n=131)与异常组(n=19)后,单因素分析结果显示,两组患者年龄(Z=-2.039,P=0.041)、治疗药物(ETV/TAF)(χ2=5.092,P=0.024)、基线eGFR水平(t=4.023,P<0.001)差异均有统计学意义;多因素Logistic回归分析显示,基线eGFR(OR=0.896,95%CI:0.841~0.955,P<0.001)和治疗药物(OR=5.589,95%CI:1.136~27.492,P=0.034)是发生肾功能异常的独立影响因素。基线eGFR预测CHB患者发生肾功能异常的ROC曲线下面积为0.781,cut-off值为105.24 mL·min-1·1.73 m-2,敏感度和特异度分别为73.68%、82.44%。Kaplan-Meier曲线分析结果显示,基线eGFR≤105.24 mL·min-1·1.73 m-2组患者肾功能异常累积发生率高于基线eGFR>105.24 mL·min-1·1.73 m-2组患者(χ2=22.330,P<0.001);ETV组患者肾功能异常累积发生率高于TAF组患者(χ2=4.961,P=0.026)。随着抗病毒治疗启动,ETV组和TAF组患者eGFR均降低(F=5.259,P<0.001),但仅在48周时ETV组eGFR水平明显低于TAF组(t=-2.422,P=0.017);在基线eGFR≤105.24 mL·min-1·1.73 m-2和基线eGFR>105.24 mL·min-1·1.73 m-2的两组患者中,eGFR亦下降(F=5.712,P<0.001),且基线及第12、24、36、48周两组患者eGFR比较,差异均有统计学意义(t值分别为-13.927、-9.780、-8.835、-9.489、-8.953,P值均<0.001)。 结论 对于ETV或TAF初治的CHB患者,ETV抗病毒治疗48周时的肾损伤风险高于TAF治疗。 Abstract:Objective To investigate the influence of entecavir (ETV) versus tenofovir alafenamide fumarate (TAF) on renal function in previously untreated patients with chronic hepatitis B (CHB). Methods A retrospective analysis was performed for the clinical data of 167 previously untreated CHB patients who received ETV or TAF treatment for at least 48 weeks at the outpatient service of Department of Infectious Diseases in The First Affiliated Hospital of Nanchang University from September 2019 to November 2023, and according to the antiviral drug used, they were divided into ETV group with 117 patients and TAF group with 50 patients. In order to balance baseline clinical data, propensity score matching (PSM) was used for matching and analysis at a ratio of 2∶1, and the two groups were compared in terms of estimated glomerular filtration rate (eGFR) and the incidence rate of abnormal renal function at week 48. According to eGFR at week 48, the patients were divided into normal renal function group and abnormal renal function group. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups. The multivariate Logistic regression analysis was used to investigate the influencing factors for abnormal renal function, and the receiver operating characteristic (ROC) curve was used to assess the performance of each indicator in predicting abnormal renal function. The Kaplan-Meier method was used to analyze the cumulative incidence rate of abnormal renal function, and the log-rank test was used for comparison. The analysis of variance with repeated measures was used to compare the dynamic changes of eGFR during antiviral therapy in CHB patients. Results After PSM matching, there were 100 patients in the ETV group and 50 patients in the TAF group. There were no significant differences in baseline clinical data between the ETV group and the TAF group (all P>0.05), with an eGFR level of 112.29±9.92 mL/min/1.73 m2 in the ETV group and 114.72±12.15 mL/min/1.73 m2 in the TAF group. There was a reduction in eGFR from baseline to week 48 in both groups, and compared with the TAF group at week 48, the ETV group had a significantly lower eGFR (106.42±14.12 mL/min/1.73 m2 vs 112.25±13.44 mL/min/1.73 m2, t=-2.422, P=0.017) and a significantly higher incidence rate of abnormal renal function (17.00% vs 4.00%, χ2=5.092, P=0.024). After the patients were divided into normal renal function group with 131 patients and abnormal renal function group with 19 patients, the univariate analysis showed that there were significant differences between the two groups in age (Z=-2.039, P=0.041), treatment drug (ETV/TAF) (χ2=5.092, P=0.024), and baseline eGFR level (t=4.023, P<0.001), and the multivariate Logistic regression analysis showed that baseline eGFR (odds ratio [OR]=0.896, 95% confidence interval [CI]: 0.841 — 0.955, P<0.001) and treatment drug (OR=5.589, 95%CI: 1.136 — 27.492, P=0.034) were independent influencing factors for abnormal renal function. Baseline eGFR had an area under the ROC curve of 0.781 in predicting abnormal renal function in CHB patients, with a cut-off value of 105.24 mL/min/1.73 m2, a sensitivity of 73.68%, and a specificity of 82.44%. The Kaplan-Meier curve analysis showed that the patients with baseline eGFR≤105.24 mL/min/1.73 m2 had a significantly higher cumulative incidence rate of abnormal renal function than those with baseline eGFR>105.24 mL/min/1.73 m2 (χ2=22.330, P<0.001), and the ETV group had a significantly higher cumulative incidence rate of abnormal renal function than the TAF group (χ2=4.961, P=0.026). With the initiation of antiviral therapy, both the ETV group and the TAF group had a significant reduction in eGFR (F=5.259, P<0.001), but the ETV group only had a significant lower level of eGFR than the TAF group at week 48 (t=-2.422, P=0.017); both the baseline eGFR≤105.24 mL/min/1.73 m2 group and the baseline eGFR>105.24 mL/min/1.73 m2 group had a significant reduction in eGFR (F=5.712, P<0.001), and there was a significant difference in eGFR between the two groups at baseline and weeks 12, 24, 36, and 48 (t=-13.927, -9.780, -8.835, -9.489, and -8.953, all P<0.001). Conclusion For CHB patients initially treated with ETV or TAF, ETV antiviral therapy has a higher risk of renal injury than TAF therapy at week 48. -
Key words:
- Hepatitis B, Chronic /
- Entecavir /
- Tenofovir Alafenamide /
- Kidney Injury /
- Root Cause Analysis
-
表 1 两组CHB患者PSM前后基线资料及特征比较
Table 1. Comparison of baseline clinical data and characteristics before and after propensity score matching in patients with chronic hepatitis B in the two groups
指标 匹配前 匹配后 ETV组(n=117) TAF组(n=50) P值 ETV组(n=100) TAF组(n=50) P值 年龄(岁) 35(30~44) 34(29~41) 0.239 35(30~44) 34(29~41) 0.435 男[例(%)] 72(61.54) 29(58.00) 0.668 60(60.00) 29(58.00) 0.814 BMI(kg/m2) 22.86±3.21 22.76±3.53 0.855 22.98±3.10 22.76±3.53 0.698 HBV DNA(log10 IU/mL) 5.19(3.40~7.47) 5.04(3.59~7.69) 0.461 5.55(3.37~7.68) 5.04(3.59~7.69) 0.886 HBsAg(log10 IU/mL) 3.31(2.47~3.86) 3.68(2.82~4.32) 0.043 3.39(2.94~3.95) 3.68(2.82~4.32) 0.235 HBeAg阳性[例(%)] 53(45.30) 30(60.00) 0.082 51(51.00) 30(60.00) 0.297 TBil(μmol/L) 17.5(12.1~31.9) 15.0(12.0~34.4) 0.780 17.4(12.1~31.6) 15.0(12.0~34.4) 0.881 Alb(g/L) 44.8(40.3~47.4) 42.8(38.7~46.0) 0.063 44.8(39.6~47.2) 42.8(38.7~46.0) 0.100 ALT(U/L) 51.0(28.3~118.7) 64.6(25.9~112.9) 0.474 52.6(33.1~134.7) 64.6(25.9~112.9) 0.949 AST(U/L) 35.6(27.8~77.2) 42.0(26.2~90.3) 0.650 36.9(28.5~96.5) 42.0(26.2~90.3) 0.957 GGT(U/L) 29.0(16.2~58.0) 38.0(17.5~91.3) 0.237 29.3(17.0~59.0) 38.0(17.5~91.3) 0.384 ALP(U/L) 78.0(66.0~100.6) 82.5(63.1~100.9) 0.768 79.8(70.2~103.8) 82.5(63.1~100.9) 0.833 TG(mmol/L) 1.21(0.82~1.72) 1.13(0.79~1.70) 0.631 1.15(0.82~1.67) 1.13(0.79~1.70) 0.806 TC(mmol/L) 4.19(3.45~4.77) 4.63(3.70~5.46) 0.069 4.18(3.51~4.78) 4.63(3.70~5.46) 0.104 FBG(mmol/L) 4.97(4.61~5.34) 4.88(4.40~5.29) 0.403 5.00(4.61~5.40) 4.88(4.40~5.29) 0.348 SCr(μmol/L) 66.29±12.39 64.82±15.69 0.520 66.63±12.46 64.82±15.69 0.445 eGFR(mL·min-1·1.73 m-2) 112.41±9.87 114.72±12.15 0.198 112.29±9.92 114.72±12.15 0.192 BUN(mmol/L) 4.10(3.50~4.90) 3.95(3.13~4.83) 0.164 4.10(3.50~4.90) 3.95(3.13~4.83) 0.173 Hb(g/L) 145(134~159) 143(131~155) 0.331 146(135~159) 143(131~155) 0.651 PLT(×109/L) 191±56 200±56 0.337 193±56 200±56 0.468 LSM(kPa) 7.1(6.1~10.3) 6.2(5.5~9.8) 0.113 7.1(6.1~10.3) 6.2(5.5~9.8) 0.105 肝硬化[例(%)] 10(8.55) 7(14.00) 0.286 8(8.00) 7(14.00) 0.248 注:TG,甘油三酯;TC,总胆固醇;FBG,空腹血糖;BUN,尿素氮。
表 2 ETV组和TAF组患者48周临床资料比较
Table 2. Comparison of 48-week clinical data between patients in the ETV and TAF groups
指标 合计(n=150) ETV组(n=100) TAF组(n=50) 统计值 P值 HBV DNA低于检测值下限[例(%)] 111(74.00) 73(73.00) 38(76.00) χ2=0.156 0.693 HBsAg(log10 IU/mL) 3.24(2.84~3.59) 3.27(2.95~3.58) 3.18(2.59~3.66) Z=-1.196 0.232 HBeAg阳性[例(%)] 68(45.33) 45(45.00) 23(46.00) χ2=0.013 0.908 TBil(μmol/L) 14.5(11.0~17.9) 14.1(9.8~17.5) 15.3(12.0~19.7) Z=-1.718 0.086 Alb(g/L) 45.5(43.3~47.3) 45.7(44.0~47.2) 44.5(42.3~47.7) Z=-0.833 0.405 ALT(U/L) 23.5(16.9~31.8) 23.9(16.6~32.5) 23.4(18.0~34.7) Z=-0.419 0.675 AST(U/L) 24.2(19.8~31.8) 25.3(19.6~31.9) 23.8(20.6~30.4) Z=-0.128 0.898 GGT(U/L) 21.0(14.0~34.0) 21.7(15.0~34.0) 20.5(11.8~35.3) Z=-0.818 0.414 ALP(U/L) 77.9(62.3~87.3) 77.4(62.8~88.7) 79.3(61.2~85.1) Z=-0.126 0.900 TG(mmol/L) 1.22(0.95~1.70) 1.20(0.93~1.69) 1.23(0.95~1.75) Z=-0.413 0.680 TC(mmol/L) 4.37(3.77~5.01) 4.37(3.80~5.00) 4.36(3.74~5.32) Z=-0.177 0.859 FBG(mmol/L) 5.00(4.69~5.43) 4.95(4.64~5.45) 5.11(4.71~5.39) Z=-1.012 0.311 SCr(μmol/L) 70.43±14.35 71.67±13.85 67.94±15.14 t=1.506 0.134 eGFR异常[例(%)] 19(12.67) 17(17.00) 2(4.00) χ2=5.092 0.024 eGFR(mL·min-1·1.73 m-2) 108.37±14.12 106.42±14.12 112.25±13.44 t=-2.422 0.017 BUN(mmol/L) 4.34±1.09 4.42±1.11 4.17±1.04 t=1.334 0.184 Hb(g/L) 146(135~158) 146(134~158) 147(135~159) Z=-0.036 0.971 PLT(×109/L) 195±62 191±67 203±52 t=1.224 0.223 LSM(kPa) 6.7(5.6~8.4) 6.8(5.6~8.5) 6.5(5.6~7.9) Z=-0.706 0.480 肝硬化[例(%)] 15(10.00) 10(10.00) 5(10.00) χ2=0.000 >0.05 表 3 肾功能正常组及异常组CHB患者基线临床资料及特征比较
Table 3. Comparison of baseline clinical data and characteristics between patients with normal and abnormal renal function in chronic hepatitis B groups
指标 合计(n=150) 肾功能正常组(n=131) 肾功能异常组(n=19) 统计值 P值 年龄(岁) 35(30~42) 34(29~41) 39(31~46) Z=-2.039 0.041 男[例(%)] 89(59.33) 81(61.83) 8(42.11) χ2=2.676 0.102 BMI(kg/m2) 22.91±3.24 22.95±3.18 22.63±3.75 t=0.395 0.694 HBV DNA(log10 IU/mL) 5.54(3.52~7.67) 5.56(3.59~7.73) 5.29(2.96~7.45) Z=-0.833 0.405 HBsAg(log10 IU/mL) 3.45(2.92~4.08) 3.40(2.93~4.24) 3.49(2.76~3.75) Z=0.500 0.617 HBeAg阳性[例(%)] 81(54.00) 72(54.96) 9(47.37) χ2=0.385 0.535 TBil(μmol/L) 16.8(12.0~31.8) 17.3(12.3~33.9) 13.8(9.0~24.7) Z=-1.545 0.122 Alb(g/L) 44.0(39.3~47.1) 44.1(39.4~47.1) 43.9(36.7~46.4) Z=-0.670 0.503 ALT(U/L) 54.0(32.0~129.5) 56.5(34.2~134.0) 38.8(24.4~103.0) Z=-1.503 0.133 AST(U/L) 38.7(28.4~92.8) 39.3(28.5~95.0) 34.2(24.0~77.5) Z=-1.011 0.312 GGT(U/L) 31.2(17.0~67.3) 32.0(18.0~75.0) 21.0(13.0~59.0) Z=-1.427 0.154 ALP(U/L) 80.6(67.1~102.5) 80.9(67.5~103.0) 77.5(63.7~94.9) Z=-0.848 0.397 TG(mmol/L) 1.14(0.81~1.69) 1.14(0.82~1.74) 1.03(0.74~1.48) Z=1.043 0.297 TC(mmol/L) 4.35(3.55~5.08) 4.27(3.50~4.97) 4.50(3.98~5.41) Z=-1.681 0.093 FBG(mmol/L) 4.99(4.48~5.36) 4.97(4.46~5.32) 5.00(4.52~5.43) Z=-0.576 0.564 eGFR(mL·min-1·1.73 m-2) 113.10±10.73 114.38±10.39 104.28±8.95 t=4.023 <0.001 Hb(g/L) 145(134~158) 144(135~157) 146(124~163) Z=-0.249 0.804 PLT(×109/L) 195±56 197±55 181±62 t=1.177 0.241 LSM(kPa) 6.9(5.8~10.0) 6.9(5.7~10.0) 6.9(6.1~10.6) Z=-0.774 0.439 肝硬化[例(%)] 15(10.00) 12(9.16) 3(15.79) 0.408 治疗药物[例(%)] χ2=5.092 0.024 ETV 100(66.67) 83(63.36) 17(89.47) TAF 50(33.33) 48(36.64) 2(10.53) -
[1] Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study[J]. Lancet Gastroenterol Hepatol, 2023, 8( 10): 879- 907. DOI: 10.1016/S2468-1253(23)00197-8. [2] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [3] MASETTI C, PUGLIESE N, AGHEMO A, et al. Safety of current antiviral drugs for chronic hepatitis B[J]. Expert Opin Drug Saf, 2022, 21( 7): 939- 945. DOI: 10.1080/14740338.2022.2045271. [4] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67( 2): 370- 398. DOI: 10.1016/j.jhep.2017.03.021. [5] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [6] JUNG CY, KIM HW, AHN SH, et al. Higher risk of kidney function decline with entecavir than tenofovir alafenamide in patients with chronic hepatitis B[J]. Liver Int, 2022, 42( 5): 1017- 1026. DOI: 10.1111/liv.15208. [7] KIM SE, JANG ES, KI M, et al. Chronic hepatitis B infection is significantly associated with chronic kidney disease: A population-based, matched case-control study[J]. J Korean Med Sci, 2018, 33( 42): e264. DOI: 10.3346/jkms.2018.33.e264. [8] FABRIZI F, CERUTTI R, RIDRUEJO E. Hepatitis B virus infection as a risk factor for chronic kidney disease[J]. Expert Rev Clin Pharmacol, 2019, 12( 9): 867- 874. DOI: 10.1080/17512433.2019.1657828. [9] YU YN, XU LY, XU T, et al. Efficacy and safety of entecavir for hepatitis B virus-associated glomerulonephritis with renal insufficiency[J]. Clin Exp Nephrol, 2023, 27( 8): 680- 686. DOI: 10.1007/s10157-023-02351-z. [10] MAK LY, HOANG J, JUN DW, et al. Longitudinal renal changes in chronic hepatitis B patients treated with entecavir versus TDF: A REAL-B study[J]. Hepatol Int, 2022, 16( 1): 48- 58. DOI: 10.1007/s12072-021-10271-x. [11] LEE JS, JUNG CY, LEE JI, et al. Comparison of decline in renal function between patients with chronic hepatitis B with or without antiviral therapy[J]. Aliment Pharmacol Ther, 2023, 58( 1): 99- 109. DOI: 10.1111/apt.17532. [12] RAY AS, FORDYCE MW, HITCHCOCK MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus[J]. Antiviral Res, 2016, 125: 63- 70. DOI: 10.1016/j.antiviral.2015.11.009. [13] YANG YM, CHOI EJ. Renal safety of tenofovir and/or entecavir in patients with chronic HBV monoinfection[J]. Ther Clin Risk Manag, 2017, 13: 1273- 1285. DOI: 10.2147/TCRM.S143286. [14] QI Q, YAO X, YANG GD, et al. Clinical application of recommended antiviral drugs for patients with hepatitis B virus-related decompensated cirrhosis[J]. Int J Virol, 2023, 30( 3): 248- 250. DOI: 10.3760/cma.j.issn.1673-4092.2023.03.015.其七, 姚欣, 杨国栋, 等. 推荐用于乙肝肝硬化失代偿期病例抗病毒治疗药物的临床应用现状[J]. 国际病毒学杂志, 2023, 30( 3): 248- 250. DOI: 10.3760/cma.j.issn.1673-4092.2023.03.015. [15] WANG F DA, ZHOU J, LI LQ, et al. Improved bone and renal safety in younger tenofovir disoproxil fumarate experienced chronic hepatitis B patients after switching to tenofovir alafenamide or entecavir[J]. Ann Hepatol, 2023, 28( 5): 101119. DOI: 10.1016/j.aohep.2023.101119. [16] HOSAKA T, SUZUKI F, KOBAYASHI M, et al. Renal safety and biochemical changes for 2 years after switching to tenofovir alafenamide from long-term other nucleotide analog treatment in patients with chronic hepatitis B[J]. Hepatol Res, 2022, 52( 2): 153- 164. DOI: 10.1111/hepr.13726. [17] BUTI M, MARCOS-FOSCH C, ESTEBAN R. Nucleos(t)ide analogue therapy: The role of tenofovir alafenamide[J]. Liver Int, 2021, 41( Suppl 1): 9- 14. DOI: 10.1111/liv.14848. [18] LENS S, POCURULL A. Editorial: The three tenors in HBV-TDF, TAF and now TMF[J]. Aliment Pharmacol Ther, 2022, 55( 1): 120. DOI: 10.1111/apt.16668. [19] CHON YE, PARK SY, KIM SU, et al. Long-term renal safety between patients with chronic hepatitis B receiving tenofovir vs. entecavir therapy: A multicenter study[J]. J Viral Hepat, 2022, 29( 4): 289- 296. DOI: 10.1111/jvh.13656. [20] INKER LA, ENEANYA ND, CORESH J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race[J]. N Engl J Med, 2021, 385( 19): 1737- 1749. DOI: 10.1056/NEJMoa2102953. [21] Disease Kidney: Improving Global Outcomes(KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases[J]. Kidney Int, 2021, 100( 4S): S1- S276. DOI: 10.1016/j.kint.2021.05.021. [22] PENG WT, GU HM, CHENG D, et al. Tenofovir alafenamide versus entecavir for treating hepatitis B virus-related acute-on-chronic liver failure: Real-world study[J]. Front Microbiol, 2023, 14: 1185492. DOI: 10.3389/fmicb.2023.1185492. [23] LIU LP, WU XP, CAI TP, et al. Analysis of efficacy and factors influencing sequential combination therapy with tenofovir alafenamide fumarate after treatment with entecavir in chronic hepatitis B patients with low-level viremia[J]. Chin J Hepatol, 2023, 31( 2): 118- 125. DOI: 10.3760/cma.j.cn501113-20221019-00507.刘丽萍, 邬小萍, 蔡天盼, 等. 恩替卡韦经治后低病毒血症的慢性乙型肝炎患者序贯联合富马酸丙酚替诺福韦治疗的疗效及影响因素分析[J]. 中华肝脏病杂志, 2023, 31( 2): 118- 125. DOI: 10.3760/cma.j.cn501113-20221019-00507. [24] JEONG S, SHIN HP, KIM HI. Real-world single-center comparison of the safety and efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in patients with chronic hepatitis B[J]. Intervirology, 2022, 65( 2): 94- 103. DOI: 10.1159/000519440. [25] HAGIWARA S, NISHIDA N, UESHIMA K, et al. Comparison of efficacy and safety of entecavir and switching from entecavir to tenofovir alafenamide fumarate in chronic hepatitis B: Long-term effects from a prospective study[J]. Hepatol Res, 2021, 51( 7): 767- 774. DOI: 10.1111/hepr.13650. [26] WANG L, XU X, ZHANG M, et al. Prevalence of chronic kidney disease in China: Results from the sixth China chronic disease and risk factor surveillance[J]. JAMA Intern Med, 2023, 183( 4): 298- 310. DOI: 10.1001/jamainternmed.2022.6817. [27] TANAKA M, AKAHANE T, KAWARATANI H, et al. Effects of entecavir and tenofovir alafenamide fumarate treatment on renal function in Japanese elderly patients with chronic hepatitis B[J]. Hepatol Res, 2024, 54( 3): 252- 260. DOI: 10.1111/hepr.13982. [28] BURNIER M, DAMIANAKI A. Hypertension as cardiovascular risk factor in chronic kidney disease[J]. Circ Res, 2023, 132( 8): 1050- 1063. DOI: 10.1161/CIRCRESAHA.122.321762. [29] ZOCCALI C, MALLAMACI F, de CATERINA R. Pharmacokinetic relevance of glomerular hyperfiltration for drug dosing[J]. Clin Kidney J, 2023, 16( 10): 1580- 1586. DOI: 10.1093/ckj/sfad079. [30] CHE YM, LI A, WANG L, et al. Influence of long-term use of entecavir on renal tubular function in patients with chronic hepatitis B[J]. J Clin Hepatol, 2023, 39( 6): 1313- 1317. DOI: 10.3969/j.issn.1001-5256.2023.06.010.车媛梅, 李嫒, 王亮, 等. 长期使用恩替卡韦对慢性乙型肝炎患者肾小管功能的影响[J]. 临床肝胆病杂志, 2023, 39( 6): 1313- 1317. DOI: 10.3969/j.issn.1001-5256.2023.06.010. -



 PDF下载 ( 1093 KB)
PDF下载 ( 1093 KB)

 下载:
下载: